Method diagram
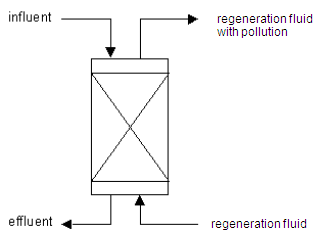
Method and installation description
An ion-exchange installation is a casing filled with synthetic resin, which is used to remove unwanted ions from a watery flow by exchanging them with less harmful ions. Besides the removal of the unwanted ions, this technique can also be used for the recuperation of valuable ions, including heavy metals.
In a strong acid cation exchanger, this resin contains sulfone groups to which natrium or hydrogen ions are bound - these are exchanged with cations in the solution. A common use for cation exchangers is the removal of heavy metals from wastewater flows by exchanging with natrium ions. In this example, the affinity of the carrier for these heavy metals exceeds the affinity of the carrier for natrium ions. Weak acid cation exchangers, with COOH as functional group, are also available.
The synthetic resin in an anion exchanger contains tertiary or quaternary ammonium groups on which hydroxide ions are positioned. These are exchanged with anions from the water flow. The removal of nitrate is an example of how anion exchangers can be implemented.
Ion exchangers only have a limited capacity, after which they become saturated and need to be cleaned. This is done by rinsing the resin with a regeneration fluid. This contains high concentrations of a regeneration product (salt, hydrochloric acid, caustic soda) with a particular pH. This shifts the balance once again and the unwanted exchanged ions return to the solution. The type of regeneration product is determined by the type of the ion-exchange installation. The ion exchanger can then be readied for use by rinsing with treated water to remove pollution residues.
To summarise, the regeneration process consists of four steps:
- the ion exchange;
- rinsing the resin in counter-flow in order to remove process water;
- regeneration of the resin with regeneration product (salt solution, acid or lye);
- rinsing the ion exchanger to remove residues of regeneration fluid.
To realise a continuous remediation process, it is best to place two or more discontinuous ion exchangers in a parallel set-up. One switches to the second bed once the capacity of the first bed has been exhausted, after which the first bed can be regenerated.
Specific advantages and disadvantages
There are various types of resin, which often have a specific impact on particular ions. This enables effective selectivity. It also enables particular heavy metals to be re-used.
Ion exchangers are quickly polluted, which considerably reduces the exchange capacity. Examples of this include pollution by micro-biology (e.g. film-forming bacteria) and pollution by suspended matter.
Another disadvantage is the relatively high operational costs for, among other things, the regeneration fluid. After use, this regeneration fluid forms a major concentrate flow that needs to be disposed of.
Application
Ion exchange is a unit process that is often implemented for the production of process water (removal of calcium, manganese, etc.). This technique has also been used in wastewater remediation for many decades, primarily as the effluent’s final purification step. The simplicity of ion exchange - in operation and installation – means that this technique has been implemented in production processes for quite some time now.
For many years, ion exchangers have regularly been used in the sector for metal surface treatments. Here are a few examples:
- Remediation and recycling of rinse water using ion exchange is used in staining and etching processes, iron phosphatation, electrolytic and electricity-free application of finishing layers. Metals could possibly be recuperated from the regeneration solution.
- The standing time of acid process baths (incl. staining and etching baths) can be extended using acid retardation. This specific use of ion exchange permits metal salts and acid to be separated, whereby the acid can be re-used in the process bath.
- Ion exchange is currently being implemented as the final remediation step for effluents, after prior removal using precipitation.
Other sectors:
- Nitrate can be removed from wastewater by exchanging with chloride. Ammonium can also be removed by exchanging with Na+. However, the latter process is less frequently implemented.
- In the graphics sector, cation exchange is used to recuperate silver from low-concentrated wastewater flows (rinse water).
Boundary conditions
The pollution of ion exchangers can be divided into pollution by soluble and insoluble substances. The resin acts as a filter for undissolved matter. These pollutants leave the resin during the rinsing and/or regeneration phase. An indicative value that can be used is a maximum suspended matter content of 10 mg/l. Dissolved matter bonds with the resin and is only released during regeneration (if the affinity of the removed matter and the resin is less than that of the regeneration product and the resin). The major pollutants are iron, oils, micro-biological matter and organic substances.
Effectiveness
Ion exchangers are implemented for the removal of cations (Cu, Ag, Au, Cd, Zn, Ca, Mg, …) and anions (nitrate, sulphate, chlorides, …).
Because ion-specific resins are often implemented, the yield generally lies between 80 and 99%. The matrix partly determines the yield.
Support aids
Salt solution, acid or lye as regeneration fluid.
Environmental issues
Rinse water and saturated regeneration fluid with harmful ions are released as residue.
Costs
The investment cost for an ion-exchange installation is divided into column costs and piping costs, on the one hand, and resin costs, on the other hand.
Operating costs are determined by the ion concentrations in the to-be-treated flow. The higher the concentration, the greater the need for frequent cleaning.
Comments
Continuous measurement is recommended to evaluate the effectiveness of an ion-exchange column. This often involves using a conductivity probe, which determines salt concentration. If specific ions need to be targeted, the water can be frequently analysed and fixed time periods can be used for regeneration.
To detect pollutants in good time, it is best to also systematically measure the pressure.
Complexity
Ion exchange is based on simple principles. However, effluent quality also needs to be followed-up correctly and rinsing frequency must be well configured.
Level of automation
Ion exchange is easy to automate.
References
- EIPPCB, Reference Document on BAT in Common Waste Water and Waste Gas Treatment / Management Systems in the Chemical Sector, draft February 2009 (revision upon release)
- VITO-SCT, revision of technical notes WASS, 2009
Version February 2010

