Method diagram
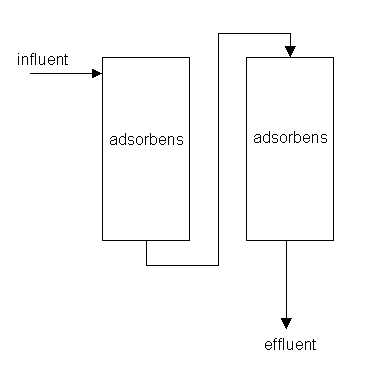
Method and installation description
Adsorption is a wastewater purification technique for removing a wide range of compounds from industrial wastewater. Adsorption is most commonly implemented for the removal or low concentrations of non-degradable organic compounds from groundwater, drinking water preparation, process water or as tertiary cleansing after, for example, biological water purification.
Adsorption takes place when molecules in a liquid bind themselves to the surface of a solid substance. Adsorbents have a very high internal surface area that permits adsorption.
Active carbon is by far the most commonly used adsorbent and is particularly suited to the removal of apolar compounds.
Other adsorbents are used for specific applications:
- Natural or synthetic zeolites (alumina-silicate-polymers)
- Have a very homogenous pore distribution and polar bonding sites. Zeolites are a lot more selective than active carbon;
- Natural clay minerals
- used for the adsorption of very polar organic and inorganic matter (ions);
- Silica gel and activated aluminium
- Very polar adsorbents with large affinity for water – normally used to remove water from an apolar medium;
- Silicic acid
Active carbon can be made from wood, charcoal and coconut. Each type is characterised by a specific surface, grain size and pore diameter. Active carbon can be used in powder form, granular form or in impregnated form.
In powder form, active carbon is added to aerobic and anaerobic wastewater purification systems or is added to physico-chemical wastewater purification processes. In this case, the added active carbon is removed and treated together with the produced sludge.
Active carbon in grains or pellets is normally used in open or closed filters. Closed filters are used in most industrial applications. They are designed so that the to-be-treated liquid is pumped through the filter and over the active carbon under pressure. Open filters are primarily used in drinking water applications, where the water flows through the active carbon beds under the force of gravity. An industrial active carbon normally consists of two columns. Both columns feature a downward flow. In time, the carbon becomes saturated and reduces the effectiveness of the filter until it stops adsorbing. After saturation, the carbon can be thermally regenerated. This takes place in a reactivation oven at high temperature. The adsorbed organic compounds are destroyed during this process. Thereafter, the carbon can be redeployed.
Active carbon can be chemically impregnated to improve its activity. Impregnated carbon was specifically developed to capture chemical substances that are normally difficult to absorb with standard activated carbon. Impregnation can be done using COH, K2CO3, H2SO4 and sulphur for the removal of organic sulphides, ammoniac and amines.
An adsorption installation with adsorbents other than activated carbon normally consists of two columns, which also become saturated in time. Zeolites and adsorbents that adsorb inorganic matter are regenerated using chemicals (e.g. NaCl solution for zeolites).
Specific advantages and disadvantages
Active carbon adsorption is a proven and much used technique because of the low energy and maintenance costs, the simplicity and the reliability. An active carbon column requires a limited amount of supervision and maintenance.
There is less experience with adsorption using other adsorbents, than there is for active carbon adsorption.
The effectiveness of the adsorption treatment is determined by the type of substance to be removed. Substances with a high molecular weight and low water solubility are better adsorbed with active carbon. The concentration of the to-be-removed substance, the presence of other organic components, temperature, pH and the set-up design also influence the effectiveness of adsorption. Realistic load factors for active carbon vary between 10-30%. The load factor for other adsorbents is lower (1-5%). This means a large column and large adsorbent quantities are needed. This results in high investment and operational costs. An advantage of using other adsorbents is that they are more specific and remove other substances than active carbon.
Application
Active carbon filtration
Adsorption with active carbon is often used as tertiary purification for the removal of organic micro-pollutants and COD, and metals in organic complexes to a lesser extent, from wastewater.
The adsorption factor is determined by various groups and compounds in the to-be-removed substances. Here are a few general rules, in decreasing adsorption factor per series:
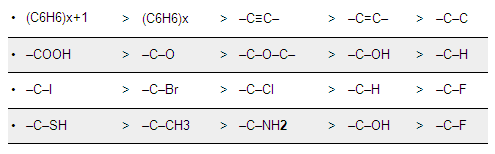
Here are a few examples of how the technique is used, though this is not a comprehensive overview:
- Active carbon adsorption is often used for groundwater purification in soil remediation, for the removal of BTEX, chlorinated hydrocarbons and PAH. This technique is particularly favoured for the purification of low volumes or for temporary (short) pumping;
- In textile distribution, active carbon is used for the removal of difficult to degrade compounds, like colorants;
- In the chemical processing industry, active carbon filtration is used for the removal of toxic substances (pesticides) from partial flows, including preventing the biological wastewater purification system from being restricted.
- Active carbon adsorption can also be used for the removal of PER from wastewater from the dry cleaning sector.
Other adsorbents
Other adsorbents are used for relatively low concentrations and when selectivity is required. An example of this is the use of zeolites for the removal of iron, ammonium, nitrate, manganese or heavy metals.
Boundary conditions
To prevent the filter from becoming blocked, the suspended matter content in the influent must be kept to a minimum, preferably no higher than 1 mg/L. Thus pre-filtration must first take place. Desorption is possible in decreasing influent concentrations, whereby the effluent adsorbs an increasing amount of pollution from the column.
Ions like iron, manganese, calcium, carbonate...can precipitate in the column and greatly decrease the absorption capacity. Pre-treatment is thus recommended.
The quantity of specific components that a column adsorbs is determined by the type of adsorbents, pollution, concentration and the temperature. Laboratory tests are a fairly accurate way of determining the effectiveness in advance.
Effectiveness
Active carbon adsorption can be implemented for removing the following parameters (removal yield indicated in brackets):
- BTEX (80-99,9 %);
- PAH (98-99,8 %);
- AOX (>90 %);
- COD;
- Colorants;
- Alcohols, xylenes;
- phenols (60 – 90 %);
- Zeolite adsorption;
- NH4-N (flows with maximum 40 mg/l, 99%).
Support aids
Active carbon, adsorbents
Environmental issues
Active carbon must be regularly generated at a high temperature. If this is not economically viable, the active carbon must be destroyed in an incinerator.
Other adsorbents must also be regenerated or, if this is not possible, processed elsewhere.
Costs
The cost prices vary greatly and are determined by the applicable discharge norms, the loading level and the volume. The price of active carbon varies from 1.28 €/kg for brown coal to 2.06 €/kg for regenerated active carbon. An average of 0.1 €/kg is calculated for the disposal of activated carbon in a landfill and 0.5 €/kg for disposal of chemical waste. The technique is clearly more expensive if there are high influent concentrations or if the loading level of the activated carbon is low. Average total costs can vary between 0.05 and 4 €/m³ treated water.
Comments
/
Complexity
/
Level of automation
An adsorption column is normally fully automated and requires a minimum amount of supervision.
References
- BAT for executing soil remediation projects and in earth cleaning centres, VITO, 2007
- EIPPCB, Reference Document on BAT in Common Waste Water and Waste Gas Treatment / Management Systems in the Chemical Sector, draft February 2009 (revision upon release)
- VITO-SCT, revision of technical notes WASS, 2009
- www.chemviron.be
- www.desotec.be
- www.zeolite-products.com
Version February 2010

