Method diagram
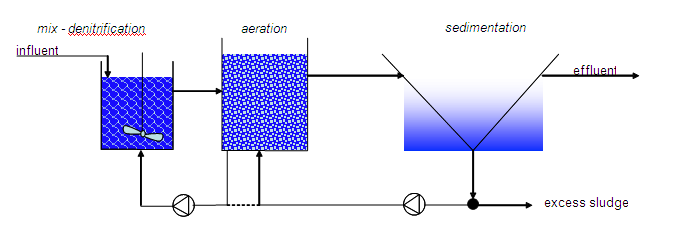
Method diagram 1: Conventional system for nitrogen removal
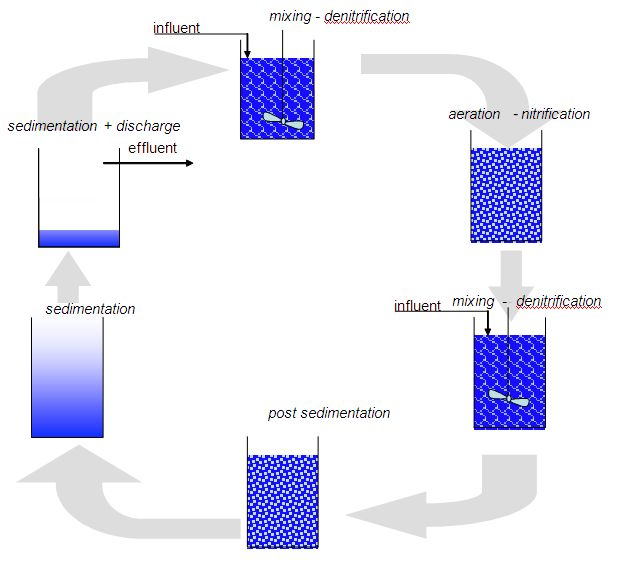
Method diagram 2: SBR type for nitrogen removal
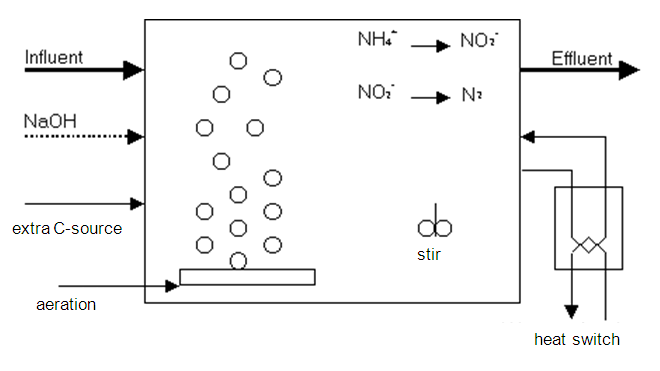
Method diagram 3: Sharon process for nitrogen removal (Single tank reactor for high activity ammonia removal over nitrite). Phases are examined in sequence.
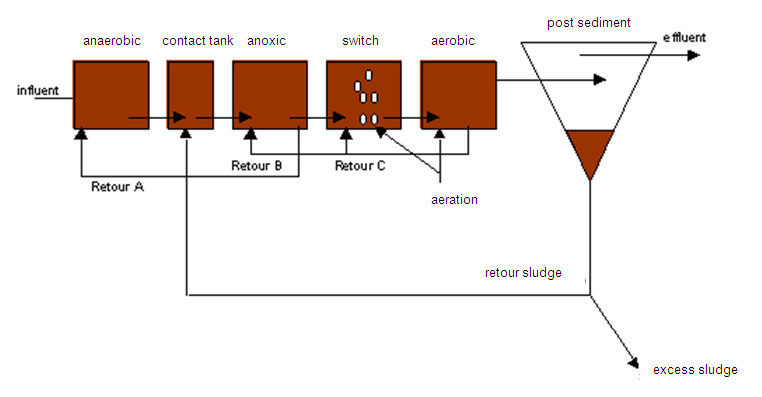
Method diagram 4: Conventional system for nitrogen and phosphorous removal (UCT, University of Cape Town)
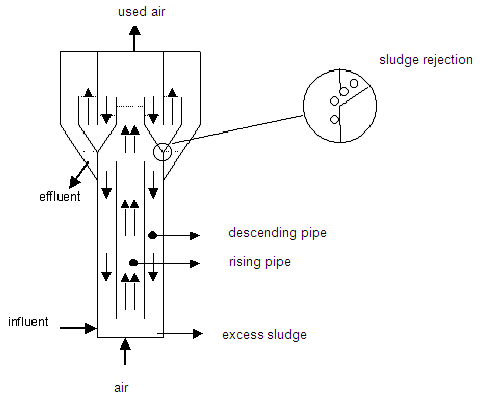
Method diagram 5: Airlift reactor
Method and installation description
Aerobic water purification based on biological oxidation is primarily used for the removal of the organic fraction in wastewater. Besides the removal of this organic matter, such systems are also able to eliminate nitrogen (N) and phosphorous (P). This can be done in active sludge systems as well as reactors with sludge as carrier. Increased nutrient removal is possible by modifying the configuration and the process.
Nitrogen
Nitrogen is an essential generator of bacteria growth and will thus be used for the production of biomass. In proportion to COD removal, intake by bacteria is as follows: per 100 g of removed COD, one can expect between 3 and 5 g of nitrogen to be removed.
If a nitrogen concentration higher than the above amount is present in the wastewater, an alternative metabolism can be induced – namely dissimilative nitrogen removal. This is a combination of a nitrification step, where ammonium is converted into nitrate, and a denitrification step, which then reduces the nitrates to nitrogen gas. The reactions can be explained as follows;
Nitrification: NH4+ + 2O2 -> NO3- + H2O + 2H+ + energy
This requires autotrophic bacteria (e.g. Nitrosomonas, Nitrobacter) and aerobic conditions. However, it should be noted that, in addition to the classic theory of nitrification by Nitrosomonas and Nitrobacter, there are increasing signs that other micro-organisms also play a role in the conversion of ammonium to nitrate.
Denitrification: 4NO3- + 5 CH2O + 4H+ -> 2N2 + 5CO2 + 7H2O + energy
This again requires heterotrophic bacteria, the presence of a carbon source and anoxic conditions (mixed and not aerated). Stoechiometrically, 2.86 g BOD is sufficient for the reduction of 1g nitrate. However, in practice, a BOD/N ratio of at least 4 or 5 will be required.
In a continuous active sludge system, denitrification is introduced by placing a preliminary denitrification basin (also see method diagram). The fresh wastewater is then fed into this basin. The nitrates are formed in the later aeration basin and are, via a system involving recirculation of the sludge/water mix, returned to the denitrification basin. Basin dimensioning is based on the nitrogen concentration in the wastewater and the COD/N or BOD/N ratio.
Integration into an SBR configuration is also possible (also see method diagram 2). This involves interchanging the mixing + supply and aeration phases in order to introduce the nitrogen process.
The aim of the Sharon process (also see method diagram 3) is to treat wastewaters containing high levels of nitrogen, like sludge rejection waters (= water released in sludge drainage), percolates from disposal sites or composting systems and specific industrial wastewaters with a high nitrogen content.
The process is carried out in a fully mixed tank, whereby the sludge is continuously drained of the effluent. This means sludge growth must equal the speed at which the sludge is drained. The process continues at high temperatures (25-39°C) and high conversion speeds. The Sharon process stimulates nitrification to nitrite instead of to nitrate. This process is referred to as nitritation. Under normal conditions, the growth of Nitrobacter sp. is not a limiting factor, whereby nitrite does not accumulate further to nitrate but is oxidised. However, at high temperatures, the growth rate of Nitrosomonas sp. will exceed the growth rate of Nitrobacter sp.. At specific retention times, it is possible to keep Nitrosomonas sp. in the reactor and to discharge the Nitrobacter sp.. This reduces the oxygen requirement by 25%. On the other hand, increased temperatures make it more difficult to introduce oxygen to the sludge/water mix. Further, the denitrification of nitrite requires 40% less carbon than denitrification of nitrate.
The aim of this approach is to reduce nitrogen from, for example, 1000 to 100 mg/l, and not to realise complete nitrogen removal. The technique is only suitable for very specific wastewaters and requires the reactor to be well designed. One should attempt to realise a correct balance between sludge growth, retention time in the reactor and sludge drainage.
The encountered reactions are as follows:
Classic nitrification : NH4+ + 2 O2 à NO3- + H2O + 2 H+
SHARON : NH4+ + 1,5 O2 à NO2- + H2O + 2 H+
(25 % O2 saved)
Classic denitrification : 6NO3- + 5 CH3OH à 3N2 + 6 HCO3- + 7 H2
SHARON : 6NO2- + 3 CH3OH à 3N2 + 6 HCO3- + 3 H2
(40 % carbon saved)
The Sharon system consists of a single reactor, where aerated and anoxic phases (with addition of BOD) are carried out alternately. During the aerobic phase, a high oxygen concentration (3 mg/l) is needed for the nitrification activity. Nitrification is an acidic process and denitrification must be started to correct the pH as soon as a critical pH value is reached. This is done by adding methanol to the anoxic phase. The pH could also be corrected by adding lye.
The Annamox process (anaerobic ammonium oxidation) is a biological process for the removal of ammonium from wastewater, whereby ammonium with nitrite is converted into nitrogen gas. The reaction is as follows: NH4++ NO2-+ -> N2 + 2 H2O.
This reaction requires an ammonium/nitrite ratio of 1:1.3. Bicarbonate acts a C-source for cell construction. During the process, around 10% of the ammonium is converted into nitrate, with the remaining part being directly converted into nitrogen gas. The optimum conditions for such bacteria are a pH between 7 and 8.5 and a temperature of 30-37°C. The problem is that these bacteria grow extremely slowly, with the doubling time amounting 7 to 11 days. Thus it takes a lot of time to start up a reactor; plus the recovery period after a process disruption is also sizeable.
Wastewater or partial flows rarely contain nitrite. This can be formed in the first step of the Sharon process (nitritation). This process can be managed so that a part of the ammonium is converted, whereby the correct ammonium/nitrite ratio can be realised in the effluent. A second reactor can then be used for the Annamox process. Such a combined Sharon/Annamox process uses less oxygen and carbon and less sludge is produced. The Canon process is a variant of this combined process with two reactors. The ‘completely autotrophic nitrogen removal over nitrite process’ combines partial nitrification with the Annamox process in a single reactor, and is primarily used for industrial wastewater. In the presence of low oxygen concentrations and surplus ammonium, it appears as if both types of bacteria can cohabit in floccule-form, with the Annamox bacteria selecting the anaerobic centre.
The process could be suitable for wastewater flows with high ammonium content (>100 mg/L) and low organic matter (C/N< 0.15).
The first full-scale Annamox reactor dates from 2002. In 2008, the Canon process was still in the lab phase.
Finally, the airlift reactor (method diagram 5) is a specific reactor concept for a nitrifying active sludge system. The reactor consists of two concentric pipes; air is blown into the inner tube, which causes the wastewater and the air to circulate. The air escapes at the top of this pipe, whereby the downward flow is fairly air-free.
Phosphorous
In addition to nitrogen, phosphorous is the second main nutrient for the growth of biomass. Consumption is determined by COD removal. For every 100 g of COD removal, one can expect a phosphorous intake of approximately 0.8 g.
Similarly to the dissimilative nitrogen removal process, it is also possible to realise far-reaching phosphorous removal. This metabolism is referred to as the ‘luxury uptake’ and requires alternating anaerobic and aerobic zones during biological water purification. The removal ratio of COD/P can thus be improved up to 100/4. The process is based on the principle that certain bacteria are capable of storing large quantities of dissolved (ortho) phosphate in their cells as undissolved polyphosphate. The anaerobic zone is not the same as an anaerobic water purification method. In biological phosphorous removal, aerobic sludge is circulated over a non-aerated zone. One achieves anaerobic conditions once the present nitrates have also been reduced (anoxia). In these conditions, specific species of bacteria (including Acinetobacter) switch to a survival metabolism whereby short fatty acids are stored in the cell as reserve material. Simultaneously, the cell’s own polyphosphate grains are degraded and released in the form of phosphate. When these bacteria then encounter aerobic conditions, they will turn to the internal carbon source. Energy released during the degradation of reserve material in the aerobic phase is used to form new cell material and to remove phosphate from the water phase once again. The phosphate, which enters with the wastewater, is thus taken in by phosphate-accumulating bacteria. Eventually, the phosphate is disposed together with the excess sludge after the aerobic phase.
Various system configurations are possible, in which aerobic, anoxic and anaerobic reactors are implemented in different ways. One example is the UCT method (also see method diagram 4).
Specific advantages and disadvantages
Active sludge systems for nutrient removal are flexible, robust and cost effective, and are often used for the treatment of both household and industrial wastewater.
Biological nutrient removal requires the main process parameters (sedimentation, load, etc.) to be supervised and followed up on a regular basis. In most cases, the process set-up can be greatly optimised.
Application
Biological aerobic purification with nutrient removal is implemented on a large scale for industrial and household wastewater with organic pollutants.
Most of the experience with active sludge systems with nutrient removal has been built up in household water purification. Most current systems have a capacity of approximately 50,000 to 100,000 i.e. Experience in industrial water purification has primarily been built up within the food industry.
The Sharon and Annamox processes (also see method diagram 3) are mainly implemented when treating wastewater with a high nitrogen content, e.g. 1000 mg/l. The aim is to realise 90% removal, and not complete N-removal. They are used for sludge rejection waters that are released during sludge drainage. The method can also be used for percolates from disposal sites or composting systems, and specific industrial wastewaters with high nitrogen and low BOD/N ratios.
Boundary conditions
For an effective nitrification process, the following process requirements must be fulfilled:
- The concentration of dissolved oxygen must amount to at least 2 mg/l;
- Due to the slow growth of the Nitrosomonas and Nitrobacter species, sludge retention in the system must be at least 10 days;
- The temperature must be between the range of 10 to 40°C, with an optimum of approximately 30°C. Nitrification takes place slower at low temperatures;
- The pH must be neutral. Very acidic (pH<5) or alkaline conditions (pH>9) must be corrected. The optimal pH for nitrification is 7.5, with nitrification being inhibited at pH values lower than 7.0. Due to the acidifying nature of the nitrification reaction, special attention must be paid to this phenomenon. Denitrification already causes a partial increase in pH.
- The content of ammoniac (NH3) and nitrous acid (HNO2) must be within a particular range, in order to prevent the inhibition of nitrification.
The following must be taken into account for an effective denitrification process:
- Denitrification occurs at temperatures of 5 to 60°C and a pH between 6 and 8;
- The content of dissolved acids should be as low as possible;
- Sufficient carbon must be present. As a rule of thumb, a theoretical COD/N ratio of at least 3 is used to enable complete denitrification. In practice, this is more likely to be 4 or 5. With regards to the content of organic carbon (C) and nitrogen (N), a sufficient amount of phosphorous (P) must be present in the wastewater. P must be added in some cases. High concentrations of salts or other chemicals can have a negative impact on the process;
- The denitrification process is clearly less sensitive to pH and temperature fluctuations and the presence of toxic components.
Phosphorous removal is subject to following:
- In the design phase, water purification methods should ideally include biological phosphorous removal. In a conventional configuration, a pre and post anaerobic tank, with the correct dimensions, can deliver positive results. The introduction of an anaerobic phase in an SBR type (also see method diagram 2) water purification system requires the various phases to be closely followed up. The mixed phase must be long enough to also realise anaerobic conditions after the anoxic conditions. This can be followed-up by measuring the redox potential of the sludge/water mix.
- Excess sludge with a high phosphorous content, which has been taken from the aerobic phase, must be stocked aerobically. If there is insufficient aeration, there is risk of phosphates being released and flowing back to the water purification system due to overflow of the thickener.
- Often in full-scale water purification systems, where it is difficult to introduce anaerobic conditions or where the COD/P ratio is lower than 100/4, iron is added to form the chemical precipitate, iron-phosphate. This is separated in the sediment via co-precipitation with the biological sludge.
Effectiveness
When purifying industrial wastewater, it can be possible to realise nitrogen and phosphorous removal in excess of 95%. The maximum biological phosphate removal amounts to 50 mg/l. If higher yields are needed, precipitation techniques like coagulation/flocculation and electro-coagulation must be used.
When purifying household wastewater, it is easy to realise effluent concentrations of 10 mg/l Ntotal and 1 mg/l Ptotal. In many cases, it is possible to realise effluent concentrations of 5 mg/L Ntotal and 0.3 mg/l Ptotal by optimising the process parameters and closely following-up the process.
The removal yields for ammonium, when treating sludge fermentation water using the Sharon process, lie between 80 and 98% for a retention time of 1.3 to 1.8 days.
Support aids
It may be necessary to add nutrients like phosphorous in order to realise the required C/N/P ratio. A rule of thumb is that BOD/N/P = 100/5/1. It may also be necessary to correct the pH of the influent using lye or acid. It may be necessary to add organic carbon for the denitrification, in the form of methanol, for example.
Environmental issues
Sludge is released as by-product. Depending on the influent, the location and the system, it may be necessary to take measures against odour problems, the emission of harmful substances and aerosols into the air, and noise problems.
Costs
The costs are comparable to those for active sludge systems. Extra aeration capacity for nitrification, stirrers and automatic valves are accompanied by extra investment costs. In a number of cases, extra carbon must be added for the denitrification step.
The investment costs for a Sharon reactor (also see method diagram 3) with a nitrogen load of 827 kg/day and a volume of 32 m³/hour, amounts to approximately € 1.600.000 (2008 data). Energy consumption is approximately 756,000 kWh per year.
The investment costs for an Annamox reactor with a nitrogen load of 300 kg/day and a volume of 35 m³/hour, amounts to approximately 1.100,000-1.200,000 €. Energy consumption is approximately 105-120 kWh per year.
Comments
In biological systems with nutrient removal, various set-up configurations are possible for the aerobic, anoxic and anaerobic zones.
Complexity
A biological purification system with nutrient removal is very complicated. Intensive follow-up of the purification process, and frequent analysis of the various locations in the purification process, is needed to manage and control the process.
Level of automation
Biological nutrient removal systems feature far-reaching automation. Analysis results must be used to optimise process management.
References
- EIPPCB, Reference Document on BAT in Common Waste Water and Waste Gas Treatment / Management Systems in the Chemical Sector, draft February 2009 (revision upon release)
- Koot A.C.J., Treatment of wastewater, Waltman Publishers, Delft (NL), 1980
- Starkenburg W., Rensink J.H. and Rijs G.B.J, Biological P-removal: state of the art in the Netherlands, Water Science and Technology, 27, 5-6, 317-328, 1993
- Stowa, The active sludge process – possibilities and limitations, 2007
- Stowa , Sharon-Annamox-Systems, 2008
- VITO-SCT, revision of technical notes WASS, 2009
Version February 2010