Principle diagram
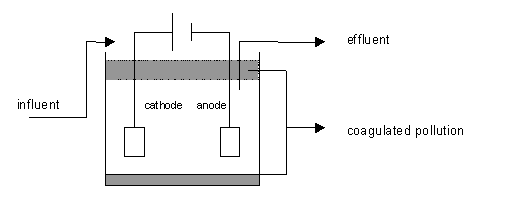
Principle and installation description
The aim of electrocoagulation is to form precipitates and compounds between colloids so these substances can be easily separated. Coagulant release is realised by electrolytically dissolving an electrode (anode, normally Fe or Al). When the electrode is dissolved, gas is released (O2, H2) which results in a flotation effect. If necessary, a (support) flocculent can be added to improve the flotation yield.
The following electrolytic reactions occur in the water:
- At the cathode :
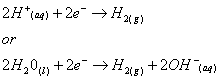
- At the anode :
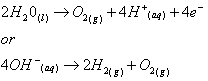
In its simplest form, an electrocoagulation reactor consists of an electrolytic cell with an anode and a cathode. If they are connected to a power supply, an oxidation reaction will take place at the anode (positive), while a reduction reaction will take place at the cathode (negative).
Specific advantages and disadvantages
Advantages:
- Easy installation and operation
- The formed silt floccules are bigger, more stable and easier to dewater than classic physico-chemical silt (see technical file W15).
- In comparison with physico-chemistry, the effluent of an EC will contain fewer dissolved organic substances
- It is possible to remove very small colloidal particles by using the implemented electrical field
- The formed gas bubbles will push the light silt upwards, where it can be easily removed.
- The EC process contains no or very few moving parts and is operated electronically, whereby maintenance costs (other than cleaning and replacing electrodes) are fairly low.
- No chemicals are needed; coagulant is released by anode reactions. As a result, chemical overdose is not possible.
Disadvantages:
- The anode is dissolved in a wastewater flow because of the oxidation, and must thus be replaced on a regular basis.
- The cathode is subjected to passive reactions (precipitation from reduced metals and hydroxides) that, as time passes, increase the resistance of the cell and thus reduces the yield.
- Depending on the conductivity and present pollution, the electrical capacity required in the cell could rise significantly.
Application
Sectors in which electrocoagulation is used include paint, gloss, varnish and printing-ink production, and in surface treatments for metals.
Boundary conditions
Minimum conductivity in wastewater is required, otherwise salts can be added which are accompanied by additional operational costs.
Effectiveness
Electrocoagulation can be implemented for the removal of:
- Settleable, suspended and dissolved substances;
- Suspended substances and colloidal particles by destabilising surface loads;
- Animal and plant oils and fats;
- Organic compounds (BOD and COD);
- Nutrients (phosphates);
- Heavy metals in the form of oxides or insoluble Fe or Al precipitates (e.g. As, Cd, Co, Cr (VI), Cu, Hg, Mo, Ni, Pb and Zn);
- Inorganic salts (e.g. CN-).
- Complex organic molecules, e.g. colorants (oxidation).
Further, electrocoagulation can be implemented for:
- Breaking-down oil-emulsions in water;
- Inactivation of bacteria, viruses and cysts.
Although a certain drop in dissolved COD is observed in many cases, this technique is not particularly suited to the removal of dissolved organic molecules, though it is suited to the removal of heavy metals, emulsions and colloids. Removal yields for metals, emulsions and colloids are comparable to those of a classic physico-chemical treatment (see technical file W17, being compiled).
Support substances
In principle, no support substances are added unless to increase conductivity. Active substances (coagulants in the form of Fe or Al) are released via anodic reactions and react with the present pollution.
Environmental aspects
Wastewater with flocculated pollution is released as waste. The silt is more compact and easier to dewater than in classis physico-chemical treatment (with coagulants and flocculants).
Costs
Average costs are equal to or higher than comparable techniques (e.g. coagulation, flocculation). For the treatment of wastewater with electrocoagulation, one must take into account a minimum cost of 0.15 €/m³ for large installations. These costs primarily consist of electricity use and, to a lesser degree, the replacement of electrodes.
For an installation of 1 m³/h with a load of ± 200 mg metals/l, one should take into account an investment cost of 150 000 €. In this case, energy use amounts to 1 kW/m3.
Case 1:
To treat a concentrated wastewater flow (Ptotal 1500 ppm) of 4 m³/day one should consider an investment of 100 000 €, without silt treatment and disposal. The removal yield for P amounts to 80%. The total treatment cost amounts to 15 €/m³
Case 2:
Wastewater of a company that chromium-coats metal surfaces. The to-be-treated wastewater volume amounts to 250 m³/yr. Energy use is 0.8-1 kW/m³, and total treatment cost is 19 €/m³. Waste concentrations of heavy metals after treatment, are less than 0.2 ppm, thus the water can be reused.
Comments
Eelectroflotation (see new technical file, being compiled) could possibly be implemented after electrocoagulation. During electroflotation, electrolysis is used to split H2O into H2 and O2. This involves the creation of gas bubbles, which ensure flotation. This approach is suited to small-scale systems, and when electricity is cheap.
Complexity
Although the process itself is very simple, potential reactions could be complex and difficult to predict. In general, the more difficult the wastewater matrix (amount and type of present pollutants), the more complex the reactions and the more unpredictable the result. Lab tests are needed to test the applicability of the technique.
Level of automation
The process can be fully automated.
References
- AEA Technology, Manual of Effluent Process Technology, Environmental & Process Engineering Department, Harwell (GB), 1991
- Beagles A., Electrocoagulation – Science and applications, Cal-Neva Water Quality Research institute (May 2004), 2004
- TNAV, supplier survey, 2008
- VITO-SCT, review of technical files WASS, 2008
- Wazutec Water treatment, Zwolle (Netherlands) : www.wazutec.nl
Version February 2010

