Method diagram
Method and installation description
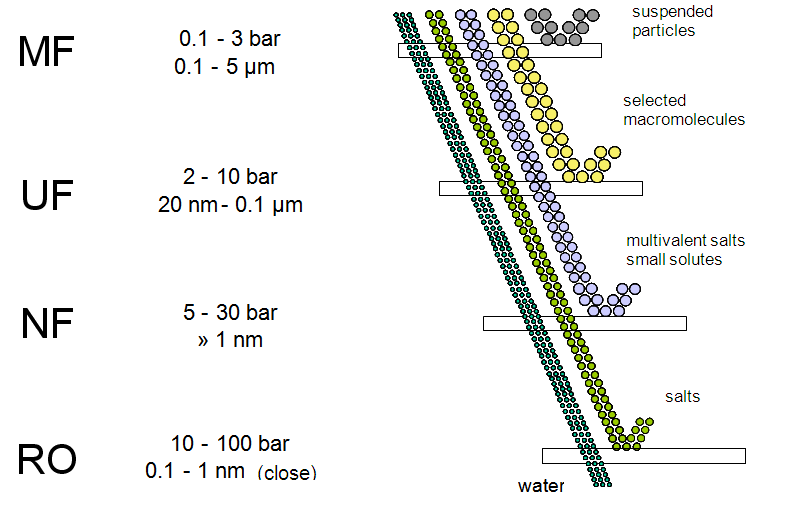
Ultra-filtration (or UF in short) is one of the pressure-driven membrane processes. The ultra-filtration process uses a membrane – a simple permeable material – which, in the case of ultra-filtration, only allows particles smaller than 20 nm to pass through it. The pore size varies between 20 nm and 0.1 microns.
Ultra-filter membranes are offered in various configurations by suppliers, with each configuration having a specific use and accompanying advantages and disadvantages. Possible membrane configurations include:
- Pipe-shaped membranes: capillary, hollow fibre or tubular;
- Plate-shaped membranes: flat plate or spiral.
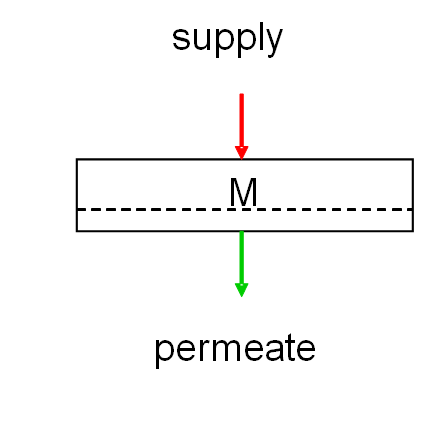
In addition to the specific membrane configurations, one can also identify a few set-ups. The 2 most commonly used methods are dead-end and cross-flow set-ups. The names refer to the way in which the supply is sent to the membrane. In dead-end UF, the supply is sent directly to the membrane. The pollution layer will thus form on the supply side of the membrane surface. This layer contains all particles that have been separated on the basis of their size (sieve effect). This layer is periodically rinsed away by briefly re-sending the produced liquid (permeate) through the membrane in an opposite direction to the production flow. This helps to loosen the hardened layer, and makes it ready for disposal. This is referred to as a semi dead-end set-up.
This re-rinsing may not be enough to remove the layer from the surface if the hardened layer is too strongly compressed or if the bond with the membrane is very strong. In this case, chemical cleaning must be implemented with, for example, bleach, peroxide, acid and alkali or detergent.
The figure below shows a dead-end set-up, where M = the membrane.
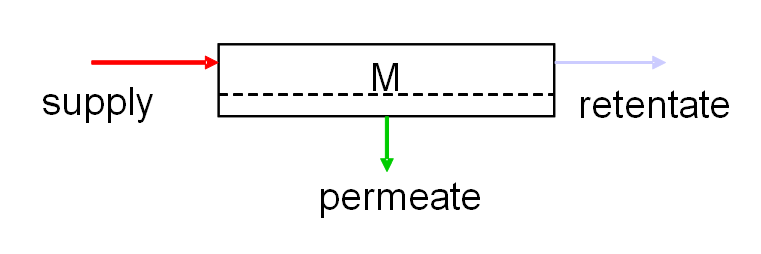
In a cross-flow set-up (see figure below), the liquid is passed along the membrane surface at a particular speed. The permeate is able to pass through the membrane and the larger particles are left behind in a concentrated flow (the retentate). The hardened layer in this set-up is continuously removed by the cross-flowing supply flow.
Specific advantages and disadvantages
The advantages of UF are:
- Low operating pressure required (higher than MF);
- Lower energy consumption than nano-filtration or reverse osmosis;
- Few manual actions required;
- Relatively cheap;
- Good permeate yield depending on the supply water and membrane choice;
- Disinfection through removal of bacteria. To a certain extent, UF allows viruses, phage, colloids and macro molecules to be removed.
Possible disadvantages of UF are:
- Only removes suspended matter and bacteria;
- Sensitive to oxidative chemicals (e.g. nitric acid, sulphuric acid, peroxide and persulphate in high concentrations); NaOCl exposure determines the life-span of the membrane and is typically 150.000 to 500.000 ppmh[1] and pH dependent;
- Damage may occur when trying to prevent hard and sharp particles > 0.1 mm;
- Membrane damage at pressure > 3 bar.
Application
UF is extremely suitable as pre-treatment in the preparation of drinking water or process water. It has excellent properties for removing suspended matter and bacteria. Further, UF is implemented in the:
- Dairy industry;
- Food industry;
- Metal industry (oil/water emulsion separation);
- Textile industry;
- Pharmaceutical industry;
- Chemicals industry;
UF is also implemented:
- As pre-treatment for nano-filtration of reverse osmosis;
- For applications involving re-use of wastewater flows.
A membrane bioreactor combines biological purification with ultra-filtration or micro-filtration.
Boundary conditions
Membranes must be protected against hard particles larger than 0.1 mm. They can be removed by regular pre-filtration. Further, supply flows, pH conditions and temperature conditions must be compatible with the membrane material.
Effectiveness
UF can be implemented for removing the following parameters:
- Suspended matter (>99%);
- Harmful micro-organisms (e.g. bacteria, protozoa, algae, fungi) (>99%).
- PAHs.
Further, UF can also be implemented to break down emulsions.
Support aids
Support aids like bleach, peroxide, acid, alkali or detergent can be used to chemically clean the UF installation. Further, iron trichloride is used as coagulant for fine suspended matter. An extra advantage of this that a very porous hardened layer is formed, which allows water to pass through.
Environmental issues
The concentrate from UF primarily consists of suspended matter and bacteria. It can be discharged together with wastewater if the accumulated concentration of suspended matter does not breach the norms. Rinse waters after chemical cleaning contain substances like bleach, peroxide, acid and alkali. These rinse waters can only be discharged to specific waste purification systems.
Costs
A typical UF installation able to process 650 m³/h costs +/- € 2.000.000.
A typical UF installation able to process 64 m³/day costs +/- € 56.000.
Comments
/
Complexity
/
Level of automation
/
References
- Bonnélye V., Guey L. and Del Castillo J., Desalination, Volume 222, Issues 1-3, 1 March 2008, Pages 59-65, 2008
- EIPPCB, Reference Document on BAT in Common Waste Water and Waste Gas Treatment / Management Systems in the Chemical Sector, draft February 2009 (revision upon release)
- Mulder M., Basic Principles of Membrane Technology, Kluwer Academic Publishers, Dordrecht (NL), 1996.
- Dutch Membrane Guide, version 2.0, 1996
- Scholz W. and Lucas M., Water Research, 37 (2003) 1859-1867, 2003
- TNAV, supplier survey, 2008
- VITO-SCT, revision of technical notes WASS, 2008
Version February 2010
[1] e.g. 15,000 ppmh means the membrane can cope with a free chlorine concentration of 1 mg/l for 150,000 hours or 17 years or of 10 mg/l for 15,000 hours or 1.7 years.

