Method diagram
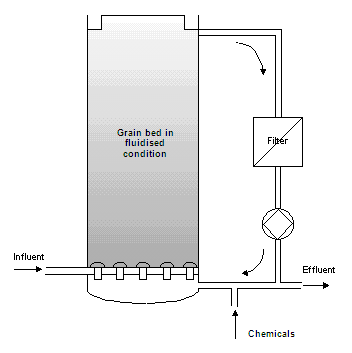
Method and installation description
In a crystallisation reactor, inorganic components (ions) that are dissolved in water are removed by forming precipitation on a suitable carrier material. To realise this, a reagent is added which forms an insoluble salt with the to-be-removed ion. The chemical reactions are equal to those of traditional chemical precipitation, though the implementation is different.
The grain reactor consists of a cylindrical column, partly filled with suitable seeding material (normally filter sand), on which germ and crystal growth takes place. The water is pumped upwards through the reactor, at a speed that keeps the grain bed in fluidised condition. A generous amount of the reagent is also added at the bottom. Complete crystallisation takes place quickly because of the large contact area of the fluidised bed. The grains grow gradually and then sink to the bottom due to their weight. Grains are regularly drained and fresh seeding material is added. The effluent is normally partly circulated. Depending on the type of wastewater and ion, low quantities of suspended matter may be produced. In this case, a filter must be placed in the recirculation flow.
Specific advantages and disadvantages
Due to the process conditions in the grain reactor, the to-be-removed ions crystallise directly in the grains’ crystal grid, so that relatively pure, almost water-free grains are produced (water content 5-10%), which are suitable for reuse. There are almost no other by-products (pollutants), like organic substances, suspended matter and other ions. This is a specific advantage compared to precipitation, where bulky floccules are formed that contain all additional pollutants, whereby reuse is normally ruled out. The crystallisation reactor is a very compact system. The main disadvantage is that crystallisation is a relatively new technique which has not been used extensively in industrial settings. The cost of chemicals is relatively high, but can be compensated for if one has a useful purpose the crystals.
Application
Crystallisation, in the form of grain reactors, is currently implemented for softening tap and process water. However, crystallisation in a grain reactor can be widely implemented; the elements Li, K, Mg, Ca, Sr, Zr, V, Cr, Mo, Mn, Fe, Co, Ni, Cu, Ag, Zn, Cd, Hg, Al, Sn, Pb, Te, F and the compounds CO3-, NH4+, PO43- and SO42- have already been successfully recuperated in a grain reactor.
Sectors in which the grain reactor is implemented, include:
- Crystallisation of metal salts from highly concentrated solutions (process baths), whereby the crystalline fraction can be reused in the company itself or in the ore processing industry;
- Wastewater from the chemicals and ore processing industry, which contains Hg, Zn, Cu, Ni, Cr, Mn, Cd, Ag and Fe, can also be treated.
- The removal of fluoride in the form of CaF2 crystals. Only free fluoride ions can be removed; bound fluoride ions are not removed.
- Removal of phosphate as calcium, magnesium, magnesium-ammonium or potassium-magnesium phosphate; the effluent concentration is determined by the selected crystal; with calcium phosphate, the effluent concentration amounts to less than 0.5 mg/l P. An example is the removal of phosphate via crystallisation from anaerobically purified wastewater from the food industry (potato processing).
Boundary conditions
With the exception of suspended matter, trivalent irons and high concentrations of intense surface-active substances, the pollutants present in the wastewater barely disturb the crystallisation. Wastewater with concentrations of 10 to 100,000 mg/l can be treated. Fluctuations in the input concentration can be addressed by modifying the circulation ratio. It is best to keep the hydraulic load of the crystallisation reactor between 40 and 75 m/h. The diameters available for full-scale reactors are 0.4 to 3.5 meters. The optimum pH is determined by the compound that is crystallised.
Effectiveness
Process conditions can normally be configured to allow the desired metal or anion to be selectively removed or a few metals or anions to be removed in combination. The lower limit for removal is determined by the solubility product of the crystallising compound.
Support aids
A suitable reagent must be selected for each type of ion, so that crystals can be formed.
Environmental issues
Only grains are drained from the reactor. It is sometimes possible to reuse the crystallised material.
Costs
A case study for the implementation of a grain reactor for fluoride removal from a flow with a volume of ~10 m³/hour, indicates an investment cost of € 500.000. Energy consumption amounts to 220 kWh.
Comments
None
Complexity
The system is relatively straightforward. The choice of process conditions and an accurate process follow-up are critical in guaranteeing effectiveness (effluent quality and quality of crystals).
Level of automation
The system can be fully automated.
References
- Baeyens J., Hosten L. and Van Vaerenbergh E., Wastewater purification, Environment Foundation - Kluwer Editorial, 1995
- EIPPCB, Reference Document on BAT in Common Waste Water and Waste Gas Treatment / Management Systems in the Chemical Sector, draft February 2009 (revision upon release)
- Giesen A., Crystallisation process enables environmental friendly phosphate removal at low costs, in Environmental Technology, Vol. 20, Issue 7, pp 769-775, 1999
- VITO-SCT, revision of technical notes WASS, 2009
- http://water.dhv.com
Version February 2010

