Method diagram
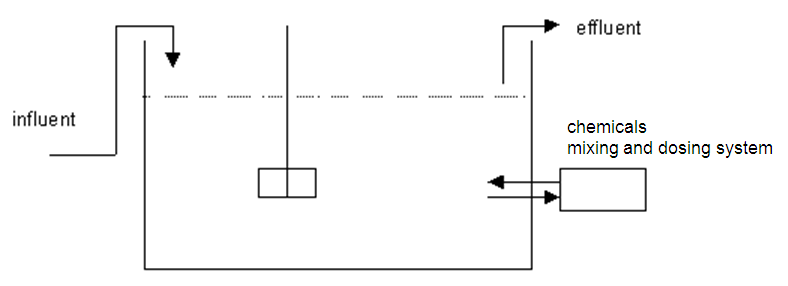
Method and installation description
The aim of precipitation is to precipitate the chemical from dissolved substances in the wastewater by adding a reagent, which forms an insoluble compound with the to-be-separated matter. Positive ions such as (heavy) metals, but also negative ions like phosphates and sulphates, can be removed via precipitation. In general, precipitation occurs in a 1 on 1 mole ratio; in other words, one molecule of dissolved matter (for example SO42- present in the form of well soluble natrium sulphate) with 1 molecule of reagent (for example, barium derived from soluble barium chloride) forms an insoluble precipitate (barium sulphate in this case). However, a certain amount of over-dosage is needed for complete removal.
Other examples include the softening of water with milk of lime (removal of Ca and Mg), removal of phosphates from wastewater using iron chloride by forming difficult to dissolve iron phosphate and the removal of heavy metals such as chrome and nickel using natrium sulphide (forming of metal sulphides). Other heavy metals can be precipitated as hydroxide by increasing the pH. Once a substance has been precipitated, it can be separated from the main stream using filtration, flotation or sedimentation. A polymer is often added to improve silt separation.
Specific advantages and disadvantages
Precipitation is a proven, relatively simple and effective technique. Precipitation can be used to obtain good results with a number of substances that are difficult to remove with other techniques. Another advantage of this technique is that very specific components can be removed, while not removing other substances; thus there is a high degree of selectivity. In some cases, waste materials from other processes can act as reagents; examples of this include iron-based silt or hydroxide (fluid or as silt). Due to the 1 to 1 ratio, a large quantity of reagent is generally needed, which is often very expensive (like, for example, in the case of barium sulphate). Another disadvantage is the large quantity of silt that is produced. Few problems are encountered if the silt can be precipitated as a useful by-product; if, for example, the silt contains heavy metals, it will be regarded as dangerous waste and will be accompanied by high processing costs.
Application
Precipitation is the most well known and most commonly used technique for the removal of metals and some anions from wastewater. Precipitation applications can be found in every sector where metals are found in the wastewater. However, it is noticeable that alternative solutions are currently being selected. The reasons for this include the relatively high effluent concentrations, the disruptive influence of surface-active substances and complex formers, and the rising cost of silt removal.
Here are a few typical applications.
- Surface treatments on metals: Milk of lime precipitation or, in extreme cases, sulphide precipitation for end-of-pipe wastewater purification. If milk of lime is implemented, then anions, fluoride, phosphate and sulphate can be removed simultaneously.
- In addition to end-of-pipe purification, precipitation can also be integrated into the process for, for example, metal recuperation in rinse water from electrolysis processes or Fe removal from flux baths in thermal galvanisation.
- Precipitation is a suitable technique for the recuperation of silver from concentrated solutions (fixing baths) in the graphics sector.
- Precipitation is a contemporary technique for wastewater purification when processing mineral products, including for the removal of metals and fluoride.
Boundary conditions
A major boundary condition is the chemical composition of the water. If a particular substance is selected, possibly co-precipitation, one should examine the optimum pH value, which substances will also be precipitate and which substances may influence precipitation.
Precipitation does not work, or works poorly, when disruptive ions are present, like complex formers. Complex formers are substances that form a well-soluble stable complex with the to-be-precipitated substances. The reagent that is added is not strong enough to break the complex bond and forms its own bond. Well know examples of strong complex formers with metals are ethylene diamine tetra-acetate (EDTA) and nitrilotriacetate (NTA). If the right quantity of EDTA is present, copper, for example, will not be precipitated. In addition to complex formers, stabilising substances also have a negative effect on precipitation, due to the ‘threshold effect, for example. A stabiliser forms a bond somewhere on the crystal structure, whereby the further growth of crystals is hindered.
The effectiveness of precipitation can be determined fairly accurately in advance, using laboratory tests.
Effectiveness
Precipitation has a high yield. The attainable end concentration is determined by the compound’s solubility product. It is difficult to determine an end concentration for combinations of pollutants, due to the interaction of substances with each other. Attainable end concentrations for single metals with Ca(OH)2 as reagent are 1-10 mg/l and approximately 0.1-1 mg/l for copper, lead, silver and cadmium with S2-.
Support aids
Chemicals as reagents; examples include iron chloride, polyaluminium chloride, milk of lime, iron hydroxide and natrium sulphide.
Acid and/or base often need to be added for pH correction.
Environmental issues
Chemical silt is formed as a by-product, whereby the dry-matter content is very important for a potential follow-up step. Silt from heavy metal precipitation is regarded as dangerous waste.
Costs
The costs for this technique are greatly determined by the choice of reagent. The level of dosage is determined by the quantity of material to be precipitated.
Case 1: Study of a company active in the metal sector (2008).
Precipitation of the metals Cr and Mo with Fe(SO4) and polymer from a wastewater flow of 3 m³/hour.
Investment costs will be € 545.000 including reactor, building, silt buffer tank, filter compressor, electro-mechanical equipment.
Operating costs excluding depreciation: € 37.000 per year including maintenance, personnel costs, chemicals and silt disposal (incineration)
Comments
Disruptive substances can negatively influence the process. Be careful for the forming of harmful complexes or harmful by-products (like, for example, hydrogen sulphide).
Complexity
Precipitation is a relatively simple process.
Level of automation
The process can be well automated.
References
- AEA Technology, Manual of Effluent Process Technology, Environmental & Process Engineering Department, Harwell (GB), 1991
- Baeyens J., Hosten L. and Van Vaerenbergh E., Wastewater purification, Environment Foundation - Kluwer Editorial, 1995
- EIPPCB, Reference Document on BAT in Common Waste Water and Waste Gas Treatment / Management Systems in the Chemical Sector, draft February 2009 (revision upon release)
- Metcalf & Eddy, Wastewater Engineering: treatment, disposal, reuse, McGraw-Hill, 1991
- TNAV, supplier survey, 2008
- VITO-SCT, revision of technical notes WASS, 2009
Version February 2010

