Method diagram
Method and theoretical description
The aim of chemical oxidation is to oxidise organic pollutants to less dangerous or harmless substances. In the best case scenario, complete oxidisation of organic substances will result in CO2 and H2O. This technique can also be used to remove inorganic components (e.g. oxidisation of cyanide). Chemical oxidisation can also be used in combination with biological purification. In this case, we refer to partial oxidisation. The purpose of chemical oxidisation as a pre-treatment technique is to either break down difficult to degrade components and make them suitable for biological degradation or to limit sludge production by partly oxidising the sludge.
Chemical oxidisation involves adding or generating oxidants in the wastewater. A few currently used oxidants include ozone (O3), hydrogen peroxide (H2O2), natriumhypochlorite or bleaching liquor (NaOCl), chlorine dioxide (ClO2), chlorine gas (Cl2), peroxy acetic acid (C2H4O3) and pure oxygen (O2). Combinations of oxidants are also possible. The most active oxidant is hydroxyl radical (OH°). This can be formed from ozone or hydrogen peroxide after activation with a catalyst (e.g. Fe2+ in a Fenton reaction) or via UV light.
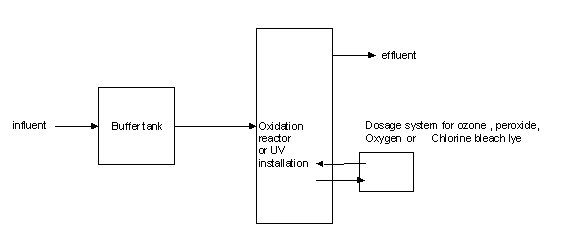
The installation for chemical oxidation consists of a buffer tank, a reactor and a dosage unit for the oxidant. This could be supplemented by a UV installation. Most oxidants are not selective, whereby prior purification (e.g. filtration step) of wastewater is often necessary.
Specific advantages and disadvantages
Each oxidant has its own advantages and disadvantages. In general, this technique requires little space. The potential risks of over-dosage must be taken into account (e.g. potential to kill a later biological purification technique). Because most chemicals are not selective, (partial) oxidisation may create products that are actually more toxic than the initial pollutants.
The disadvantage of the Fenon reaction is the pH sensitivity and the increased sludge production.
Applications
Below is a brief description of some examples of chemical oxidisation:
- Treatment of groundwater for the removal of cyanides, PAH, BTEX, phenols and other organic micro-pollutants.
- Chemical oxidisation of percolation water as post-biological purification. The aim of this is to further oxidise certain residual pollutants (persistent COD or AOX).
- Increasing biodegradability by treating the influents and effluents of a biological purification system. Besides increasing the BOD/COD ratio, potential toxic substances can be converted into molecules that are easy to degrade biologically.
- The removal of colour components (e.g. textile sector or paper industry).
- Detoxification of galvano-effluents.
- Treatment of cooling water (AOX reduction and biological growth).
Boundary conditions
There are few, or no requirements at all, for the wastewater that will be treated. A cheap pre-treatment (e.g. removal of Fe from groundwater) will sometimes be carried out to restrict the costs of chemical oxidisation.
An important point of note in the use of UV light is the turbidity and colour of the wastewater. The turbidity reduces the permeability of the UV light and the colour may adsorb the UV light. Therefore, in order to achieve the required purification yield, a more intensive light may be necessary. It is thus important for suspended components, amongst other things, to be removed from the wastewater in advance. This can be done easily using sand filtration, for example.
Testing is used to determine the most optimum process conditions. The parameters that play a role in this are the type of oxidant, the required dosage, the acidity and the retention time in the reactor.
Effectiveness
Chemical oxidisation is primarily implemented for the removal of persistent organic substances (e.g. dioxins, pesticides and biocides), organic compounds (e.g. BOD and COD, AOX, EOX, TOC, TOX, BTEX (benzene, toluene, ethylbenzene and xylene, MAH, phenols and PAHs), nutrients (nitrogen and organophosphorous compounds) and inorganic salts (e.g. CN-, S-2 and SO3-2).
In the case of recalcitrant COD and colour components, there are no known limits for the treatment of input concentrations. Both parameters can be removed up to 100%.
In general, the yield from chemical oxidisation is good to excellent. Screening should indicate whether this technology can be used for a particular case, possibly with prior treatment steps. The intended yield can also be realised by possibly increasing the oxidant dosage. However, when doing so, a trade-off must be made with the overall cost price.
Support aids
Besides the above mentioned oxidants (see method and theory description paragraph), catalysts (Fe2+) can also be added.
Environmental issues
A certain amount of extra energy is needed for the introduction or generation of oxidants.
The end products of chemical oxidisation are oxidised pollutants, on the one hand, and residual concentrations of oxidants or their decomposition products, on the other hand (e.g. acetic acid in the use of peroxy-acetic acid as oxidant). These are discharged along with the treated wastewater.
Before the wastewater is discharged, one must ensure that excessive amounts of oxidising substances are not present.
Costs
The costs can be determined by various factors, such as volume of wastewater, type and concentration of the pollutant, presence of disruptive components, desired yield, etc.
For ozone, the following assumptions can be used to estimate the operating costs:
- Energy consumption for production of O3: Based on pure oxygen, the costs will be 6 to 15 kWh/kg O3, based on air this amounts to 17 to 30 kWh/kg O3
- Energy cost price: 0.06 €/kWh
- Produced ozone concentration: 8 - 10% (10 to 12 kg O2 / kg O3)
- Oxygen cost price: 140 €/tonne
The required dosage of ozone must be determined via experiments. A typical ozone dosage is 2 kg ozone/kg COD. The operational costs for energy and oxygen will, depending on the required dosage, be 1 to 2 €/m3.
Considering the limited stability, ozone must be produced on-site. For an ozone generator with a capacity of 1.5 kg ozone/h, one should consider an investment cost of € 100.000. The contact tank and piping must be able to resist oxidising conditions.
The dosage of liquid oxidisation, like H2O2, requires low investment costs. If activation with UV is also needed, the investment costs will be considerably higher. For the oxidisation of a wastewater flow of 1 m3/h and 5.000 mg COD/l, the investment costs for UV lamps amount to approximately € 65.000.
Comments
Ozone that is left behind after ozone generation can be reused in biological wastewater purification, for example.
Complexity
The complexity of the technique is partly caused by the need to correctly dose oxidants and possibly catalysts.
Level of automation
Automation is possible but requires effective follow-up.
References
- EIPPCB, Reference Document on BAT in Common Waste Water and Waste Gas Treatment / Management Systems in the Chemical Sector, draft February 2009 (revision upon release)
- TNAV, supplier survey, 2008
- TNAV, Academia Meets Industry meeting day; Theme: advanced oxidation processes (AOPs), Sint-Katelijne Waver, 04/11/2008
- VITO-SCT, revision of technical notes WASS, 2008
Version February 2010