Description
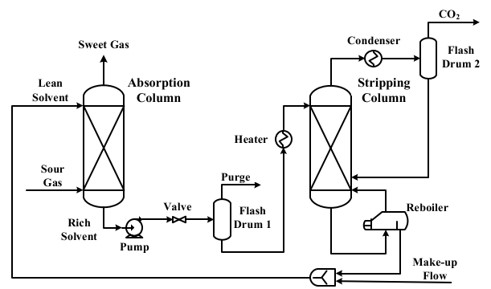
TECHNICAL ASPECTS (all % are volume-based)
Point sources: Power, Steel, Cement, Refining & Petrochemical, Waste to Energy, BECCS, Pulp & Paper, Gas Processing, & Industrial Flue Gases.3
CO2 concentration range: >5%3
CO2 capture efficiency: 80-95%3
CO2 purity: 99%3
Min. feed gas pressure: 20 bar2
Max. feed gas temperature: 120 °C2
Typical scale: Large (> 100,000 tCO2/yr)4
Primary energy source: Thermal (steam) and electricity
Impurity tolerance: Solvents will absorb impurities such as NOx, SOx, and mercury, and form stable salts, resulting in near zero emissions (<100 ppm).5
FUNCTION IN CCU VALUE CHAIN
- Capture CO2 from flue gases.
- Removal of H2S, avoiding any additional purification step.
- SOx and NOx form potassium salts when reacted with K2CO3, which are easily recoverable, avoiding the use the additional flue gas pretreatment steps.
LIMITATIONS
- High energy consumption due to the regeneration step
- HPC absorbs CO2 slowly, requiring large absorption columns. Additives help, but don't reduce the size significantly. Increasing CO2 partial pressure can improve efficiency but adds energy-intensive turbomachinery.6
- Requires pressurized flue gas.
- Corrosion and solvent degradation issues over time due to impurities.
ENERGY
- Heat (steam) is primarily used for the regeneration of the solvent in the stripping column.
- Electricity is mainly used for pumping, fans, and control systems.
CONSUMABLES
- Potassium Carbonate (K2CO3) is the primary solvent used in the absorption column to capture CO2.
- Water for preparing the potassium carbonate solution and for controlling the concentration of the solvent.
- Steam is used in the regeneration step.
| Parameter | Value |
|---|---|
| Heat (GJ/tCO2) | 2.177 – 2.55 |
| Electricity (kWh/tCO2) | 7707 |
| Cooling water (t/tCO2) | 34.57 * |
| K2CO3 make-up (kg/tCO2) | 0.128 |
|
7 solvent conc. - 40 wt.%; CO2 conc. – 30 %; CO2 capture efficiency – 87%; includes only flue gas compression to 10 bar; excludes CO2 compression. 5 UNO MK 2 technology. * Cooling water is reused. |
|
COSTS
CAPEX: 24 €/tCO2 9
Main CAPEX: absorption column, strippers, and heat exchanger.
OPEX: 60 €/tCO2 9
Main OPEX: electricity, fixed OPEX.
CO2 capture cost: 84 €/tCO2 9
55 €/tCO2 7
CO2 avoidance cost: 104 €/tCO2 avoided9
9 CO2 Capsol technology; CO2 conc. – 5%; CO2 capture efficiency – 85%; 1.92 Mt/yr; operating hours – 7446 hr/yr; 2025 euros; discount rate – 7.8%; lifetime – 25 yrs; electricity – 162 €/MWh; steam – 18 €/MWh; steam for regeneration produced by heat recovery; cost includes flue gas cooling, compression to 9 bar, CO2 capture, compression to 101 bar and purification; 39% share in CAPEX for capture technology.
7 Solvent conc. - 40 wt.%; CO2 conc. – 30 %; CO2 capture efficiency – 87%; includes only flue gas compression to 10 bar; excludes CO2 compression; K2CO3 – 1235 €/t; cooling water – 0.012 €/t; electricity – 47.8 €/MWh.
ENVIRONMENTAL
CO2 footprint: 152 kgCO2e/tCO2 8
(dependent on electricity source, for NGCC electricity = 60 kg CO2e/tCO2)
Spatial footprint: 11,719 m2 for 1.92 MtCO2/yr 9
(land cost – 25.6 €/m2; estimation includes flue gas cooling, compression, CO2 capture, compression and purification)
Environmental issues: Water usage for the aqueous solution, waste disposal.
ENGINEERING
Maturity: Commercial (TRL 9)3
Commercial plants are operational globally.
Retrofittability: Feasible
Compatibility with existing infrastructure that already handles flue gas streams, such as hydrogen production plants, refineries, ammonia synthesis plants, and natural gas processing facilities.
Scalability: High
Suitable for a wide range of industrial applications, particularly for medium to large-scale plants.
Process type: Liquid solvent-based with chemical reactions.
Deployment model: Centralized or Decentralized.
Decentralized CO2 absorption at point sources with centralized desorption.
Technology flexibility: Hybridization with other capture technologies is feasible. Other technologies, such as membranes, can be used upstream to increase CO2 concentration.
INNOVATIONS
- Enzymatic Carbon Capture: Enzymatic carbon capture technology by CO2 Solutionsᵀᴹ (Saipem) efficiently reduces CO2 emissions, meeting regulatory standards. It operates using low-grade residual heat at 85°C, integrating easily with waste heat sources to save costs and minimize environmental impact. The technology uses a non-toxic carbonate solvent, producing minimal hazardous byproducts, ensuring environmental safety.
- Heat integration: Capsol’s integrated post-combustion carbon capture and heat recovery system uses the efficient and safe HPC (Hot Potassium Carbonate) solvent, simplifying permitting. It is ideal for sectors like cement, biomass, energy-from-waste, and gas turbines.
TECHNOLOGY PROVIDERS
- Benfield™ by Honeywell, United States.
- Hot Potassium Carbonate by Sumitomo SHI FW, Finland.
- UNO MK 3 by KC8 Capture Technologies, Australia (Regeneration energy 2 – 2.5 GJ/tCO2)
- CapsolEoP® by Capsol Technologies, Norway (Regeneration energy 0.6 – 1.9 GJ/ton CO2 for CO2 concentration 4 – 20%10; 95% capture efficiency; 35 €/tCO2 capture cost; can be fully electric)
- Hot Potassium Carbonate by K2-CO2, Italy
- CATACARB® HPC by Andritz AG, Austria (Absorption temperature 80 – 100 °C; 90% capture efficiency; >99% purity)
- Enzymatic Carbon Capture by SAIPEM, Italy.
BENCHMARK
MEA-based primary amine scrubbing technology serves as the benchmark for all the CO2 capture technology due to its wider applications and high commercial readiness.
CONTACT INFO
Mohammed Khan (mohammednazeer.khan@vito.be)
Miet Van Dael (miet.vandael@vito.be)
ACKNOWLEDGEMENT
This infosheet was prepared as part of the MAP-IT CCU project funded by VLAIO (grant no. HBC.2023.0544).
REFERENCES
1. Al Rashid MR, Bousmaha B. Operating Experience Of The Benfield Carbon Dioxide Removal System At Ruwais Fertilizer Industries (FERTIL). In: IFA Technical Conference. ; 2004. Accessed February 29, 2024. https://ureaknowhow.com/wp-content/uploads/2014/03/2004-Rashid-Fertil-IFA-Operational-experience-of-the-benfield-CO2-removal-system.pdf
2. Ngu LWW, Mahmoud A, Sunarso J. Aspen Plus simulation-based parametric study of Benfield process using hot potassium carbonate promoted by diethanolamine. IOP Conf Ser Mater Sci Eng. 2020;778(1).
3. Barlow H, Shahi SSM. State of the Art: CCS Technologies 2024.; 2024.
4. Menmuir D, Florence S, Taylor K. Next Generation Carbon Capture Technology Technology Review.; 2022. Accessed February 17, 2025. https://assets.publishing.service.gov.uk/media/629493dbe90e070396c9f6a0/aecom-next-gen-carbon-capture-technology-technology-review-annex-1.pdf
5. Anderson C, Harkin T, Ho M, et al. Developments in the CO2CRC UNO MK 3 Process: A Multi-component Solvent Process for Large Scale CO2 Capture. Energy Procedia. 2013;37:225-232.
6. Navedkhan M, Lakshminarayan J, Biliyok C, Levihn F. Integration of Hot Potassium Carbonate CO 2 Capture Process to a Combined Heat and Power Plant at Värtaverket. In: International Conference on Greenhouse Gas Control Technologies. GHGT; 2022.
7. Chuenphan T, Yurata T, Sema T, Chalermsinsuwan B. Techno-economic sensitivity analysis for optimization of carbon dioxide capture process by potassium carbonate solution. Energy. 2022;254:124290.
8. Grant T, Anderson C, Hooper B. Comparative life cycle assessment of potassium carbonate and monoethanolamine solvents for CO2 capture from post combustion flue gases. Int J Greenh Gas Control. 2014;28:35-44.
9. Menmuir D, Berry K. Next Generation Carbon Capture Technology Technoeconomic Analysis.; 2022. Accessed February 17, 2025. https://assets.publishing.service.gov.uk/media/6294923ce90e07039e31b777/aecom-next-gen-carbon-capture-technology-technoeconomic-analysis.pdf
10. Staggat P. Cost-effective Carbon Capture through HPC technology licenses. In: Rotterdam Conference. ; 2023. Accessed February 17, 2025. https://fortesmedia.com/files/files/Doc_Pack/Industry_CCUS_2023/Rotterdam_Conference_Presentation_v1.pdf

