DESCRIPTION
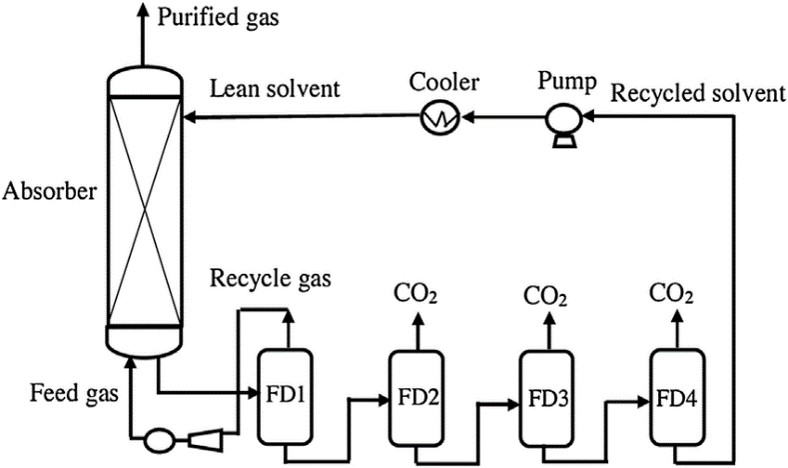
TECHNICAL ASPECTS (all % are volume-based)
Point sources: Hydrogen production, ammonia production, cement production, refining and petrochemical, and gas processing, biomass & coal gasification.5
CO2 concentration range: 20-70%6
CO2 capture efficiency: 95%3 – 99%5
CO2 purity: 98%7
Min. feed gas pressure: ~21 bar8
Max. feed gas temperature: 40 °C9
Typical scale: Medium - Very large (7 MtCO2/yr)10
Primary energy source: Electricity (& heat for 2-stage)
Impurity tolerance: Solvent is chemically stable and does not form heat-stable salts when in contact with the flue gas.11
FUNCTION IN CCU VALUE CHAIN
- Capture CO2 from flue gases.
- Most of the CO2 is recovered at higher pressures (~6 bar), greatly reducing the CO2 compressor power and costs.12
- Another acid gas, H2S, typically present in synthesis gas, is also removed but requires a thermal stripping step.
LIMITATIONS
- Have low affinity for CO2 compared to chemical solvents and therefore require more solvent.
- Process equipment, such as absorbers, pumps, and pipework, is larger.
- Weak affinity of CO2 means removing CO2 mainly from streams at moderate to high partial pressures and a smaller regeneration energy requirement.
- Selexol has a high affinity towards water, requiring an upstream flue gas dehydration step.
- Exhibits higher viscosity than those of common physical solvents, particularly at low temperatures, resulting in low mass transfer and high packing requirements.9
ENERGY
- Electricity is used by solvent pumps, recycle gas compressors, CO2 compression, and flue gas compression, if the flue gas is not pressurized.
- Electricity is used in a refrigeration system if the operating temperature is very low or sub-zero.
- Steam may be required for an upstream dehydration step since physical solvents have a high affinity towards water.
CONSUMABLES
- Selexol solvent is used to capture CO2 from flue gas with negligible solvent loss.
- Cooling water is used in the H2S stripping column to condense entrained water and solvent.
| Parameter | Value |
|---|---|
| Solvent make-up (kg/tCO2) | 0.00713 |
| Cooling water (t/tCO2) | ~12* |
| Heat (kWh/tCO2) | -NA- |
| Electricity (kWh/tCO2) | ~78* |
| *VITO TEA study (details below) | |
COSTS
CAPEX: 3 - 4 €/tCO2 12 (3.4 €/tCO2 *)
Main CAPEX: absorption column, flash drums and flue gas, and recycle compressors.
OPEX: 21 - 24 €/tCO2 12 (16.1 €/tCO2 *)
Main OPEX: electricity and cooling water.
CO2 capture cost: 24-28 €/tCO2 12 (19 €/tCO2 *)
Depends on absorption temperature, scale, CO2 concentration, CO2 partial pressure, etc.
12 IGCC plant; lifetime – 30 years; CO2 concentration – 40.4%; CO2 capture capacity – 3.08 MtCO2/yr; feed pressure = 29.6 bar; electricity price = 108 €/MWh; includes CO2 compression to 130 bar; CRF – 0.106; 2018 euros.
* VITO TEA study; IGCC plant; lifetime – 25 years; CO2 concentration – 40.4%; CO2 capture capacity – 3.75 MtCO2/yr; feed pressure = 29.6 bar; electricity price = 100 €/MWh; includes CO2 compression to 110 bar; WACC = 4.6%; 2022 euros; includes initial solvent inventory in CAPEX.
CO2 avoidance cost: 21 - 32 €/tCO2 avoided 14
14 IGCC plant; CO2 compression, transport, and storage cost included; 2000 euros; lifetime – 30 years.
Note: The CO2 avoidance cost presented here assumes a pressurized feed gas. For atmospheric pressure streams, Selexol is not economical because of low CO2 partial pressure and the significant cost of compression.
ENVIRONMENTAL
CO2 footprint: ~17 kgCO2eq/tCO2 13
Estimated using only utilities data from literature.13 Relatively low footprint due to lower regeneration energy requirements.
Spatial footprint: 178,125 m2 (475x375) for 6.02 MtCO2/yr 15 (includes two-stage Selexol process and CO2 compression system)
Environmental issues: Minimal process emissions and effluents.1
ENGINEERING
Maturity: Commercial (TRL 9)5
Most widely used method with a physical solvent.
Retrofittability: Feasible
No significant modifications are required because Selexol operates at relatively low temperatures and pressures, and no thermal regeneration is required.
Scalability: High
Multiple trains can be added for upscaling 5,7
Process type: Liquid solvent-based without chemical reactions.
Deployment model: Centralized or Decentralized.
Decentralized CO2 absorption at point sources with centralized desorption.
Technology flexibility: Hybridization with other capture technologies is feasible. Other technologies, such as membranes or PSA, can be used upstream to increase CO2 concentration.
TECHNOLOGY PROVIDERS
- SeparALL™ by HONEYWELL UOP, United States (process)
- Selexol™ by DOW, United States (solvent)
- Genosorb® by CALRIANT, Switzerland (solvent)
ALTERNATE PROCESSES
Purisol
Purisol uses n-methyl-2-pyrrolidone (NMP) solvent to remove acid gases CO2, H2S, and COS at ambient temperature. Flow schemes used for this solvent are like those used for Selexol. It is particularly well suited to the purification of high-pressure, high CO2 synthesis gas.
Fluor
Fluor processes use propylene carbonate solvent to remove CO2 and H2S from flue gases as well as C3+ hydrocarbons, COS, SO2, and CS2. It cannot be used for selective H2S treatment because it is unstable at high temperatures.
CONTACT INFO
Mohammed Khan (mohammednazeer.khan@vito.be)
Miet Van Dael (miet.vandael@vito.be)
ACKNOWLEDGEMENT
This infosheet was prepared as part of the MAP-IT CCU project funded by VLAIO (grant no. HBC.2023.0544).
REFERENCES
1. UOP. UOP Selexol TM Technology for Acid Gas Removal.; 2009.
2. Songolzadeh M, Soleimani M, Ravanchi MT, Songolzadeh R, Han -W, Rogov VA. Carbon Dioxide Separation from Flue Gases: A Technological Review Emphasizing Reduction in Greenhouse Gas Emissions. Sci World J. 2014;828131:34.
3. Technologies for Efficient Purification of Natural and Synthetic Gases |.
4. Kapetaki Z, Brandani P, Brandani S, Ahn H. Process simulation of a dual-stage Selexol process for 95% carbon capture efficiency at an integrated gasification combined cycle power plant. Int J Greenh Gas Control. 2015;39:17-26.
5. Barlow H, Shahi SSM. State of the Art: CCS Technologies 2024.; 2024.
6. Im D, Roh K, Kim J, Eom Y, Lee JH. Economic assessment and optimization of the Selexol process with novel additives. Int J Greenh Gas Control. 2015;42:109-116.
7. Nelson M, Vimalchand P, Brown R, et al. Carbon Capture at the Kemper IGCC Power Plant. GHGT 2018 - 14th Int Conf Greenh Gas Control Technol. 2018;(October):1-18.
8. Kohl AL, Nielsen R. Gas Purification. 5th ed. Gulf Pub; 1997.
9. Ashkanani HE, Wang R, Shi W, Siefert NS, Hopkinson D, Resnik K. Levelized Cost of CO2 Captured Using Five Physical Solvents in Pre-combustion Applications. Int J Greenh Gas Control. 2020;101:1-46.
10. MIT. LaBarge Fact Sheet: Carbon Dioxide Capture and Storage Project. Carbon Capture and Sequestration Technologies @ MIT. 2016
11. Zaman M, Lee JH. Carbon capture from stationary power generation sources: A review of the current status of the technologies. Korean J Chem Eng. 2013;30(8):1497-1526.
12. Xin K, Hashish M, Roghair I, van Sint Annaland M. Process Simulation and Economic Analysis of Pre-combustion CO2 Capture With Deep Eutectic Solvents. Front Energy Res. 2020;8:323.
13. Singh B, Strømman AH, Hertwich EG. Comparative impact assessment of CCS portfolio: Life cycle perspective. Energy Procedia. 2011;4:2486-2493.
14. Chen C. A Technical and Economic Assessment of Selexol-Based CO2 Capture Technology for IGCC Power Plants. Carnegie Mellon University; 2005.
15. IEA. Retrofit of CO2 Capture to Natural Gas Combined Cycle Power Plants.; 2005

