DESCRIPTION
Deoxygenation (oxygen removal) of captured CO2 stream can be performed by several technologies, including catalytic oxidation of carbon monoxide, propane, methanol, and hydrogen, oxidation of coal/carbon, cryogenic distillation, and chemisorption of oxygen on copper. A summary of these technologies is given in the literature.1 The choice of technology depends on the final oxygen limit that is acceptable for a certain CCUS technology. Based on the advantages and disadvantages, the catalytic oxidation of hydrogen was ranked first. The only disadvantage is the increase in the water content of the CO2 stream, requiring a post-dehydration step. Catalytic oxidation of hydrogen (H2) involves the use of a noble catalyst, typically palladium, to facilitate the reaction of hydrogen with oxygen, forming water (H2O) as a byproduct at an operating temperature of 80 °C. A dehydration step or a condensation step is required after the process to produce a pure CO2 stream with an oxygen concentration of less than 5 ppmv.2
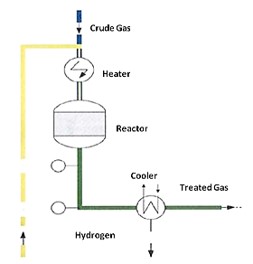
REMOVED COMPONENTS
- Primary target – Oxygen (O2)
- Indirect – Water (H2O)
FUNCTION IN CCU VALUE CHAIN
- Purification of CO2 streams.
- Preventing corrosion and material degradation.
LIMITATIONS
- Palladium catalyst is sensitive to contaminations (SOx, NOx).
- Hydrogen concentration should be stoichiometric or slightly in excess to ensure complete oxidation.
- Hydrogen concentration must be carefully controlled, typically within 0.1 to 5% for safe and efficient operation (exothermic reaction).2
ENERGY
- Electricity for blowers and compressors.
- Heat (steam) for pre-heating the catalyst bed.
CONSUMABLES
- Catalysts (e.g., platinum, palladium).
- Hydrogen for the catalytic reaction.
- Cooling water is used to cool the outlet gas stream.
| Parameter | Coal2 | NGCC2 |
|---|---|---|
| Hydrogen (kg/tCO2) | 0.006 | 0.033 |
| Catalyst make-up (m3/yr) | 1.77 | 0.42 |
| Cooling duty (MWh/tCO2) | 0.046 | |
| *All are estimated values.2 | ||
COSTS
Deoxygenation costs depend on the oxygen concentration in the CO2 stream. Higher oxygen levels require more hydrogen and a catalyst for purification, increasing overall costs.
Total purification cost: 2.7 – 4.8 €/tCO2 2 *
* includes both oxygen and water removal; lower range for coal-fired case and upper range for NGCC case; use CO2 stream compositions from the table below to convert the values to per tonne of oxygen; ignore water removal as it is expected to cost less.
2 Oxygen limit – 10 ppmv; water limit – 50 ppmv; palladium catalyst cost – 600,000 €/m3; catalyst lifetime – 5 yrs; hydrogen – 23 €/kg; plant lifetime – 25 yrs; operating hours – 7446 hr/yr; discount factor 8%; CFR 0.0937; electricity price 38 €/MWh; cooling water price – 0.24 €/m3; 2012 euros.
| Component | Coal base case | With purif. | NGCC base case | With purif. |
|---|---|---|---|---|
| CO2 (mol%) | 99.14 | 99.91 | 99.06 | 99.85 |
| H2O (mol%) | 0.774 | 50.1 | 0.774 | 48 |
| O2 (ppmv) | 67.6 | 11.4 | 317 | 10.3 |
| N2 (mol%) | 0.0756 | 0.0762 | 0.134 | 0.135 |
| Ar (ppmv) | 11.3 | 11.4 | 22.5 | 23.4 |
| CO2 flow (t/h) | 515 | 515 | 125 | 125 |
TECHNOLOGY PROVIDERS
- SILPURE® by Silica, Germany
- PURAVOC BLUE™ (catalyst) by Johnson Matthey, United Kingdom
- Puristar® R0-20 (catalyst) by BASF, Germany
- OxiGone (catalyst) by Research Catalysts, Inc., United States
- ZerO2 oxygen removal from biogas by ECOVAPOR, United States
- De.OXO Biogas® and De.OXO Hydrogen® by HyGear, Netherlands (primarily for oxygen removal (99.8%) from biogas and hydrogen with final oxygen content of <5 ppmv, but can be used for CO2 purification)
ALTERNATIVE TECHNOLOGIES
Chemisorption of oxygen on copper: In this process, oxygen is adsorbed on copper at a temperature up to 200 °C to form copper oxide. Once copper reaches its maximum oxygen-holding capacity, it is regenerated by using either H2 or CO to produce copper and water/CO2. Generally, two reactors operating alternatively in oxidation and regeneration modes are needed to ensure continuous operation. The oxygen holding capacity of one kilogram of copper is 4.5 liters of dry oxygen. This process can reduce the oxygen concentration in the range of 1 – 10 ppmv.3 The chemisorption on copper is only used to remove some ppm levels of O2.
GetterMax 100 (catalyst) by Research Catalyst, Inc., United States
Puristar® R3-11G (catalyst) by BASF, Germany
CONTACT INFO
Mohammed Khan (mohammednazeer.khan@vito.be)
Miet Van Dael (miet.vandael@vito.be)
ACKNOWLEDGEMENT
This infosheet was prepared as part of the MAP-IT CCU project funded by VLAIO (grant no. HBC.2023.0544).
REFERENCES
1. Abbas Z, Mezher T, Abu-Zahra MRM. CO2 purification. Part I: Purification requirement review and the selection of impurities deep removal technologies. Int J Greenh Gas Control. 2013;16:324-334.
2. Abbas Z, Mezher T, Abu-Zahra MRM. CO2 purification. Part II: Techno-economic evaluation of oxygen and water deep removal processes. Int J Greenh Gas Control. 2013;16:335-341.
3. Abbas Z, Mezher T, Abu-Zahra MRM. Evaluation of CO2 Purification Requirements and the Selection of Processes for Impurities Deep Removal from the CO2 Product Stream Selection and/or peer-review under responsibility of GHGT. Published online 2013.

