Method diagram
|
|
Method and installation description
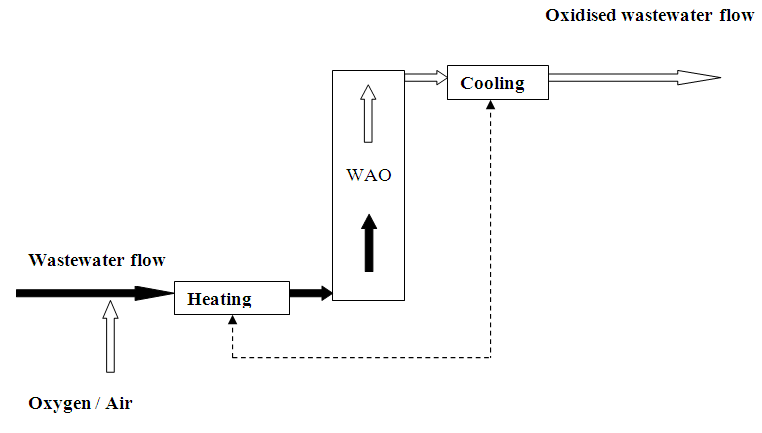
The aim of this treatment technique is to oxidise the organic pollution in a wastewater flow. The technique uses an oxygenated gas as oxidant (normally air or O2) and operates at higher pressures and temperatures. This is in contrast with ‘classic’ chemical oxidation. Implementation can be aimed at total oxidation or partial oxidation where organic substances are converted into smaller hydrocarbons that, for example, are biodegradable or can be removed in another manner. The extent of oxidation is determined by the operating conditions of the process.
3 operating areas are normally identified:
- Lower operating conditions (170-220oC; to 4 MPa);
- Higher operating conditions / Subcritical (240- 320oC; 4 to 20 MPa);
- Supercritical (> 374oC; > 22.1 MPa).
Wet Air Oxidation (WAO)
The process for lower or sub-critical process conditions is also referred to as Wet Air Oxidation. This technique is often used as a pre-treatment technique. The treatment converts the present organic substances to low-molecular, biologically degradable substances.
Super-critical water oxidation (SCWO)
If one performs the process under super-critical conditions, this is referred to as super-critical water oxidation (SCWO). This process takes place without a catalyst and aims to realise total oxidation using a treatment lasting a few minutes at best. This process also makes it possible to precipitate inorganic pollutants.
This technique has various industrial applications, which mainly differ in terms of reactor design. In some processes, a homogenous catalyst (e.g. Cu or Fe-ions) is used to improve the oxidation process and to reduce costs. Recent developments have focused on the use of heterogenic catalysts (e.g. precious metals or basic metal-oxide catalysts).
Specific advantages and disadvantages
In principle, the technique can be widely implemented.
The WAO technique offers little selectivity. The present organic substances will be oxidised to water and CO2, and intermediary oxidation products (including low-molecular alcohols, carbon acids and aldehydes, as well as ammoniac and inorganic acids and salts).
The oxidation process works at increased temperatures and pressure, which requires high energy input.
Application
Various application areas can be identified:
- Conditioning and oxidising sewer water sludge from purification installations, using WAO at lower operating conditions. The effluent is further treated biologically;
- Regeneration of active carbon;
- Treatment of organically loaded wastewater flows from the chemicals, petrochemical and pharmaceutical industries. Examples include flows containing phenols, halogenated hydrocarbons or polycyclic aromates;
- Treatment of dangerous wastewater flows;
- Treatment of spent caustic waste flows;
- Treatment of black liquor in the paper mill industry.
Boundary conditions
The oxidation process takes place at increased temperatures and pressure.
The technique is primarily suited to the treatment of waste flows with a COD of 20-200 g/l. There must be sufficient oxidisable matter to permit an auto-thermal reaction. At 250- 280oC the requirement is typically 80-100 g COD g/l to 20 g/l if the influent has already been pre-heated. The process is not appealing, in terms of energy consumption, for low organic loads. Another limitation concerns the presence of inorganic salts and Cl-, which could cause corrosion.
Some low-molecular substances, particularly acetic acid, are more resistant against oxidation and require more extreme oxidation conditions if they also need to be removed.
Effectiveness
The yield varies greatly depending on the application and operating conditions (temperature, pressure, treatment time and catalyst). When sludge is treated in mild conditions, the COD-reduction only amounts to 5 to 15%. In contrast, super-critical water oxidation of waste flows realises a COD reduction of 96.5 to 99.99% for substances like 2-chlorine-phenol, nitrobenzene, PCBs.
Support aids
The process requires oxygenated gas, like air or oxygen. Some processes require a catalyst (e.g. Fe(II)).
Environmental issues
Degradation products may be released from the pollutant as by-products. This may be in the form of emissions or undissolved matter in the effluent. The oxidant may also be released.
Costs
It is not possible to provide an exact costs overview. Treatment costs will mainly be determined by the scale, the required treatment conditions and time, and the material requirements set for the reactor (e.g. to prevent corrosion).
Both WAO and SCWO require considerable investment. In the case of SCWO, in particular, treatment costs will mainly be determined by capital taxes on investments.
Operating costs are primarily determined by the energy costs associated with pumping the influent and compacting the air or oxygen. Further, personnel costs relating to maintenance also play a major role.
The literature makes reference to costs between 10-60 €/ton (info up to 1994). For SCWO, costs between 30-100 €/ton (info 2001) have been mentioned.
Comments
None
Complexity
WAO and SCWO are complex processes. Set-ups require particular focus on stability and safety.
Level of automation
The process normally has a high level of automation.
References
- Bijsterbosch J.W., RIZA, Chemical oxidation techniques: An overview of available techniques, 1994
- Cocero M.J., High Pressure Technology : fundamentals and applications, Ed. A. Bertucco and G. Vetter, Elsevier, 2001
- Dellafontaine H., Foussard J-N, Waste Management 20, 15-25, 2000
- EIPPCB, Reference Document on BAT in Common Waste Water and Waste Gas Treatment / Management Systems in the Chemical Sector, draft February 2009 (revision upon release)
- Kolaczkowsi S.T. et al, Wet air oxidation: a review of process technologies and aspects in reactor design, Chemical Engineering Journal 73, 143-160, 1999
- Mishra V.S., Wet Air Oxidation, Ind. Eng. Chem. Res. 34, 2-48, 1995.
- VITO-SCT, revision of technical notes WASS, 2009
Version February 2010