Method diagram
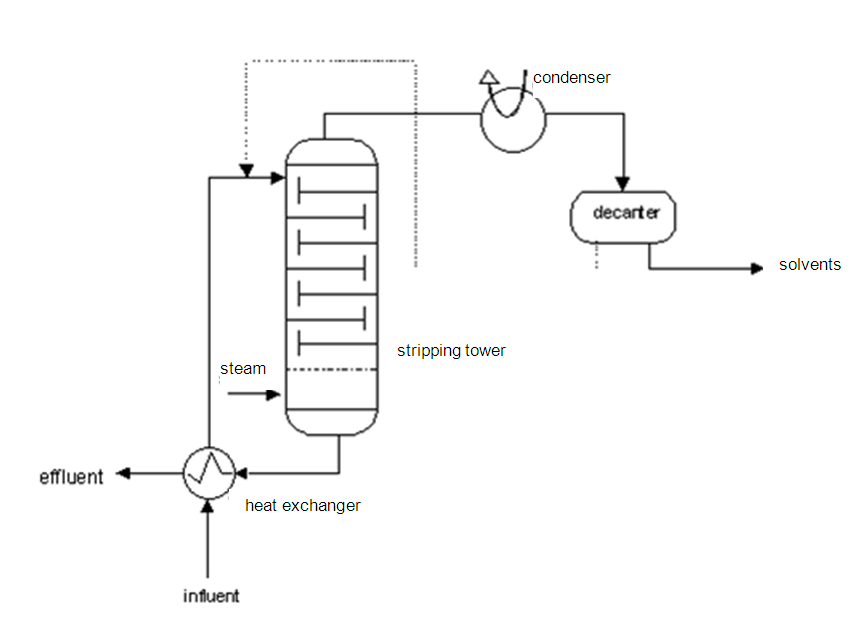
Method and installation description
Steam stripping of wastewater is a distillation process [1] where volatile organic matter is removed from water. The volatilization of organic matter is greatly determined by the temperature. Due to the higher temperature used in steam stripping, compared to air stripping, more soluble organic matter will be removed than when air stripping is implemented. No air treatment is needed; the removed components will be in a concentrated flow that can either be recuperated or must be destroyed (e.g. via incineration).
The principle is based on reducing the partial pressure of the pollution by heating and by creating a positive mass transport from the water to the gas phase via steam injection. A steam stripper consists of a supply pump with heat exchanger, a packed or dish column, a condenser with a separation drum and a reflux device, and a decanter. The wastewater must be free of solid matter, which could block the heat exchanger.
Specific advantages and disadvantages
A stream stripper selectively removes components. Substances that dissolve well in water, which cannot be removed with the classic stripping method, can also be separated from water. Steam stripping consumes relatively large amounts of energy because electricity is used. Further, lime residue is also left behind, which could pollute the stripping tower.
Application
Steam stripping is used in both inorganic and organic chemistry.
Boundary conditions
Steam stripping is implemented at temperatures between 90 and 120°C and pressures between 1 and 2 bar. The capacity of a steam stripper can vary from 1 to 100 m3/hour. The SM content must be <5 ppm.
The "stripability" of the compound is indicated by the Henry coefficient. In steam stripping for VOC, this coefficient is as follows: >10 to 100 ppmw[2].
Lime residue caused by shifts in the carbonate equilibrium must be prevented.
Effectiveness
Steam stripping is primarily implemented for the removal of volatile organic matter (incl. chlorinated hydrocarbons, VOX and BTEX), sulphur compounds (H2S, phosphine) and NH3. Stripping is normally carried out on the concentrated partial flow.
Further, typical uses include:
- Acetone removal and recuperation of wastewater;
- Removal of MEK (methyl ethyl keton) and MTBE (methyl tertiary butyl ether);
- Removal of chloroform, bromoform and other halogenated components;
- Alcohol (ethanol, propanol, isopropylalcohol (IPA), butanol) removal from water;
- Solvent recuperation or removal (tetrahydrofuran, hexane, heptane).
Steam stripping has a high removal yield. Here are a few examples of removal efficiencies:
- VOX: >99%;
- Benzene: >96%;
- Toluene: >99%.
H2S can be removed from 5.000 ppm to 20 ppm.
Support aids
Ammoniac can be stripped in a steam stripper. If ammoniac is interfering with other pollutants that need to be removed, this can be confirmed using neutralising substances.
Environmental issues
Flue gases and condensed fluid are released as by-products. In most cases, these flows require further treatment.
Costs
No concrete data is available about the cost price of the purification technique itself. The cost of the measurement and regulation equipment, e.g. for the sludge level, can be estimated as follows:
- Investment costs ± 720 €/ m³ (excl. VAT);
- Operating costs ± 50 €/ m³ (excl. VAT).
Comments
None.
Complexity
The settings for the stripping process must be established with great accuracy. It is a relatively complex process to manage.
Level of automation
No data available.
References
- EIPPCB, Reference Document on BAT in Common Waste Water and Waste Gas Treatment / Management Systems in the Chemical Sector, draft February 2009 (revision upon release)
- Environmental Technology, Monographs handbook, Envi Tech Consult, INC, Den Haag, Handbook on Wastewater, 1996.
- TNAV, supplier survey, 2008
- VITO-SCT, revision of technical notes WASS, 2008
Version February 2010
[1] Distillation is a technique using vaporisation to separate two or more substances in a solution, based on the difference in these substances’ boiling points.
[2] Parts Per Million by Weight

