Method diagram
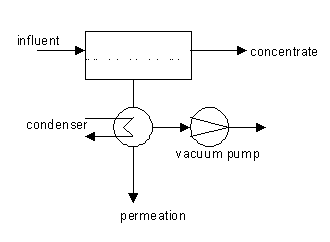
Method and installation description
Pervaporation is a membrane process comparable to distillation[1], and combines permeation and vaporisation. Pervaporation is used to separate liquid mixes. The used membrane is a dense non-porous membrane or a very finely-porous ceramic membrane that displays an affinity towards the component one wants to remove. Specifically for this process, the permeating component is converted into an evaporation phase, due to the low (partial) vapour pressure on the permeate-side. This low vapour pressure is normally achieved by placing a (slight) vacuum on the permeate-side of the membrane. In most cases, the permeate is re-condensed. The pervaporation process contains 3 steps:
- Selective sorption in the membrane on the influent-side;
- Selective diffusion through the membrane;
- Desorption in the gas phase on the permeate-side.
There is also an alternative process, referred to as vapour permeation. Vapour permeation involves the supply being introduced in vapour form before it comes into contact with the membrane. This alternative is particularly interesting if the supply comes from an earlier process step in vapour form.
Pervaporation is not based on a liquid-vapour balance like distillation, but is based on differences in sorption and diffusion in the various components in the supply. This it is an interesting alternative for separating azeotropic mixes or mixes with similar boiling points. Further, it offers an energy advantage because only the permeating component must be converted in the gas phase.
Good hydrophilic pervaporation membranes are available on the market. The use of pervaporation to drain solvents is clearly on the increase. Recently, the technique was also used to drain bio-ethanol. Good hydrophobic commercial membranes are also available on the market for removing volatile organic components from wastewater. They are also suitable for removing volatile components like hydrocarbons from air. Membranes for separating 2 organic components are still in the development phase.
Specific advantages and disadvantages
The main advantage of pervaporation is the major potential to save energy. Savings of above 50% are easy to realise. However, the membranes and membrane installations, (incl. need for vacuum) are currently relatively expensive. Thanks to considerable energy saving, the investment is still economically viable for complex distillations.
Further, pervaporation avoids contamination of the required flow, in contrast to, for example, azeotropic distillation where entrainers or extra components are used to eradicate the azeotrope.
If the supply contains suspended matter or dissolved salts, then membrane pollution may be encountered. In this case, an effective pre-treatment or vapour permeation can be implemented.
Application
Pervaporation is a practical and financially viable alternative to distillation when draining solvents, particularly where azeotropes must be degraded or liquids with similar boiling points must be separated. In such cases, a hybrid process (distillation combined with pervaporation) is normally the most economically viable.
Hydrophilic pervaporation or vapour permeation can also be used to remove water from a reaction mix and thus favourably alter the reaction-balance. This is, for example, used for esterification reactions.
To remove volatile organic substances (incl. chlorinated hydrocarbons and BTEX) from wastewater, pervaporation is competitive with active carbon adsorption (see technical files W46 and W47, under review) and stripping (see technical files W43 and W44).
Throughout the world, there are various large-scale implementations, with the majority addressing drainage of alcohol (ethanol and isopropanol). The capacity of most installations lies between 50 and 300 l/day. Thus, these are rather small installations.
Here are a few examples of sectors where pervaporation can be implemented: the chemicals industry, pharmaceutical industry, petrochemical sector, refineries, tank and drum cleaning and paint, gloss, varnish or printing-ink production.
Boundary conditions
In order to realise sufficient flux[2] during pervaporation, work is normally carried out at temperatures between 50 and 100°C. Typical fluxes averaged for a pervaporation process are around 1 kg/lhm2.
Pervaporation should be implemented to remove the minority component from a mix. In this case, the required membrane surface and required energy will be at their lowest.
Most pervaporation membranes work optimally when the permeating component in the supply is below a particular maximum level.
Components with high boiling points make pervaporation more difficult. They restrict selectivity and can block the membrane.
Effectiveness
Pervaporation is primarily used for the removal of volatile organic compounds (incl. VOX, BTEX, MAK, methyl tert-butyl ether, phenols, styrene, PAHs and apolar KWS) from wastewater. For this purpose, the technique is competitive with active carbon adsorption (see technical file W46, under review) and stripping (see technical files W43 and W44).
Pervaporation easily removes components from water to below 1%. Higher concentrate purification levels are possible but require a relatively large membrane surface, because the encountered fluxes are relatively low (low driving force).
Support products
If the correct pre-treatment is implemented (e.g. micro-filtration) in the presence of suspended matter, and if a vapour permeation process is used for the presence of salts, then no problems are expected during membrane cleaning - thus no chemicals are needed.
Environmental issues
Pervaporation is recognised as an energy-efficient alternative to difficult distillations. The concentrate is normally the product (incl. for drainage). Due to the high selectivity of the membranes, the permeate can often be discharged or re-used.
Costs
A theoretical economic evaluation of a hybrid distillation/pervaporation unit for a to-be-processed isopropanol/water mix (50/50 weight %) by Van Hoof et al. (2004) resulted in the following costs:
- Operational costs (primarily energy): ~17 €/ton product
- Investment costs: ~40 €/ton product
- Maintenance (incl. membrane replacement) ~13 €/ton product
Thus the costs, for draining solvents that form an azeotrope, using such a hybrid distillation/pervaporation process, are around half of those associated with ‘classical’ azeotropic distillation.
Considering the limited implementation, no investment costs can be mentioned for a full-scale installation.
Comments
The technique can result in major energy reductions. In the near future, this technique is expected to develop further and be implemented on a larger scale.
Complexity
Pervaporation/vapour permeation is a relatively simple process. The process parameters for process optimisation are temperature, vacuum, supply speed and supply concentration.
Level of automation
Far-reaching automation is possible.
References
- EIPPCB, Reference Document on BAT in Common Waste Water and Waste Gas Treatment / Management Systems in the Chemical Sector, draft February 2009 (revision upon release)
- Jonquières A. et al., Industrial state-of-the-art of pervaporation and vapour permeation in the western countries, Journal of Membrane Science, 5189 (2002) 1-31, 2002
- Mulder M., Principles of Membrane Technology, Kluwer Academic Publishers, Dordrecht (NL) 1996
- Van Hoof V. et al., Economic comparison between azeotropic distillation and different hybrid systems combining distillation with pervaporation for the dehydration of isopropanol, Separation and Purification Technology, 37 (2004) 33-49, 2004
- VITO-SCT, revision of technical files WASS, 2008
- Wynn N., Reactions and Separations, Pervaporation, Comes of Age, CEP Magazine, October 2001, 66-72, 2001
[1] Technique using vaporisation to separate two or more substances in a solution, based on the difference in these substances’ boiling points.
[2] Quantity of effluent filtered per membrane surface-unit and per unit of time
Version February 2010

