Method diagram
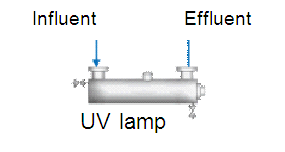
Physical (e.g. UV)
SOURCE: VITO-SCT, 2009
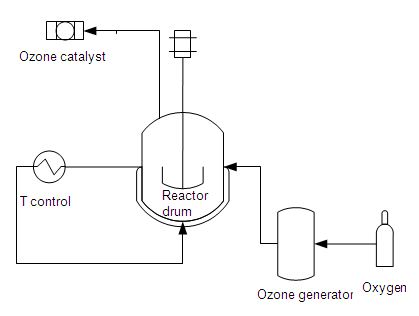
Chemical (e.g. ozone in batch)
SOURCE: VITO-SCT, 2009
Method and installation description
Disinfection can be carried out by adding an external agent to kill off harmful micro-organisms or to remove them using membranes. An effective disinfection agent must be toxic against micro-organisms, at a concentration distinctly lower than the toxicity barrier for humans and animals. It must also work quickly and be effective enough to prevent future bacteria growth. The use of membrane filters will, depending on the selected membrane and the process conditions, help to realise partial or complete removal of micro-organisms.
Techniques that use external agents can be divided into direct and permanent disinfection. Direct disinfection is sufficient if growth in the remaining micro-organisms is very slow or if there is no risk of recontamination. UV radiation and ozonisation cause such contamination. Chemical methods and electrolysis help to realise lasting or permanent disinfection. The disadvantage of some disinfection techniques - possibly in combination – is that they produce toxic by-products. Extra measures must be taken to prevent these by-products from forming or to eliminate them. New techniques include ionising radiation (electron beam), plasma technology and pulsed electric fields.
The choice of implemented technique can be influenced by numerous factors. These influential factors include:
- Composition of the water (incl. hardness, turbidity, pH, T, …);
- Need for permanent or temporary disinfection;
- Type of micro-organism;
- Safety considerations (transport & storage);
- Health considerations (humans & environment);
- Compatibility (space, integration in process,…);
- Complexity (actions etc.);
- Cost price;
- Requirements set for treated water.
The effectiveness of the disinfectant is determined by the targeted micro-organisms. Thus, the most suitable disinfectant will be selected based on the species and will also be determined by the other influential factors.
Specific advantages and disadvantages
Chemical oxidation is caused by adding an oxidisation product. The most commonly used chlorine compounds are chlorine gas (Cl2), natrium hypochlorite (NaOCl), calcium hypochlorite (Ca(OCl)2) and chlorine dioxide (ClO2). The advantages of disinfection based on chlorine are the simple installation, flexible dosage, the lasting effect and the relatively low price. The optimum chlorine dose is difficult to determine because this is greatly determined by the composition of the water. Temperature, acidity and water load are the main influential parameters. Thus it will always be necessary to overdose. Dangerous by-products can also be formed.
The use of ozone is greatly determined by the composition of pollution in the water. Further, there is continuous ozone production while pollution in the wastewater can vary greatly. Thus overdosing will always take place and excess ozone (which is toxic) must be avoided. Ozone treatment has a positive effect on the odour, taste and colour of the treated water. The installation is more complex than a unit for chlorine disinfection, but is easy to automate. Ozone reacts very quickly and does not have a delayed effect. The poor solubility of ozone in water is a disadvantage.
The yield of UV treatment is determined by the water quality (incl. turbidity), the intensity of UV radiation, the timeframe of radiation and the reactor configuration. Two types of lamp can be use for UV treatment: Low pressure (LP) lamps and medium pressure (MP) lamps. The latter have an intensity that is 15 to 20 times higher, provide faster results and have a higher penetration capacity. The disadvantages are the effectiveness at higher temperatures and the higher energy input. The main advantages of UV are the lack of impact on water composition, direct effect, limited space and safe and controlled disinfection. To-be-treated water must be clear and UV must have no depot effect.
Peroxide only has a limited disinfection effect. Thus it is also used in combination with other techniques (e.g. ozone). Peroxide leaves no by-products and dosage can be modified to suit demand.
Disinfection via membranes is normally carried out using UF membranes. A major advantage of this technique is that no chemicals are used. The harmful organisms are removed from the water flow, which is in contrast with other techniques where they are only killed.
Application
The use of disinfection can be found in numerous industrial sectors. Here are a few examples: drinking water treatment, (purified) water treatment in water re-use, decontamination of swimming pools, water treatment in cooling towers and scrubbers, water disinfection in car and truck washes, applications in the cosmetics sector, hospitals, the pharmaceutical industry…
Boundary conditions
Each technique has specific requirements for the to-be-treated water. The to-be-removed species also determine which technique will be considered. However, not every technique is effective against particular organisms. Some disinfectants alter the composition of the water, which is not always required.
Treatment with UV requires the water to be suitable for maximum UV transmission. Scaling and fouling on the UV lamp must be carefully monitored. UV is a technique which does not influence the composition of the water.
In a number of non-selective techniques (e.g. ozone and chlorination), the initial content of oxidisable compounds determines the effectiveness of disinfection. A high amount of oxidisable compounds means the amount of disinfectant required to be effective must also be high. The pH of the water also determines whether a specific oxidant can be used. Other present substances (e.g. ammonium) can greatly influence the effectiveness of the disinfectant.
Effectiveness
The yield is high if the correct disinfection technique is selected.
Support aids
Depending on technique Chlorine gas, natrium and calcium hypochlorite, chlorine dioxide, chlorine amines, ozone, peracetic acid, peroxide, metals, …
Environmental issues
By-products are left behind by some disinfectants (e.g. bromate from ozone). Due to the high dosages, residues may remain in the water. These must be avoided.
Costs
The investment cost is determined by the application, the water quality, the system and the required capacity, among other things. An installation for 46 g/h ClO2 costs ca. €10.000 (production + in-line measurement). Operating costs are primarily determined by energy consumption, chemicals and personnel costs.
Ozone must be produced on-site, which means electricity consumption is fairly high (150 g O3/kWh). Based on pure oxygen, the costs will be 6 to 15 kWh/kg O3, based on air this amounts to 17 to 30 kWh/kg O3. In an installation with a capacity of 5 m³/h, the ozone generator has an installed electrical capacity of 2 to 10 kW. The investment cost for this installation amounts to ca. € 30.000.
The investment cost for a UV installation for 10 m³/h, amounts to ca. € 6.200. The main operating costs can be attributed to the replacement of lamps, energy, chemicals for cleaning and personnel. Depending on the type of lamp, lamps must be replaced after 5.000 to 100.000 working hours. MP lamps cost 4 to 5 times more than LP lamps.
Comments
Poisonous gases may be released when chlorine and ozone are used.
Complexity
UV, and the dosage of chemicals, requires a simple installation. Careful attention must be given to the limited solubility of ozone in water. This makes these units more complex.
Level of automation
The installations can be very effectively automated, with sufficient opportunities for follow-up.
References
- VITO-SCT, revision of technical notes WASS, 2009
Version February 2010