Method diagram
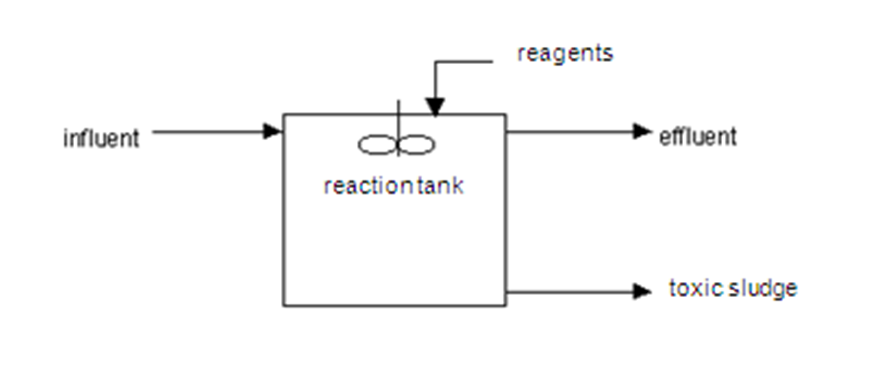
Method and installation description
Detoxification means the removal of harmful or unwanted components from wastewater. It primarily entails the removal of specific inorganic waste materials from wastewater, using redox reactions. In practice, this involves a number of specific cases, e.g. chromate detoxification (Cr2O7-2, CrO4- and CrO3), cyanide detoxification (CN-), nitrite detoxification (NO2-) and detoxification of sulphur compounds (S-2, S2O3-2 and SO3-2).
Reduction of the inorganic component is often needed before the correct chemicals for water purification can be dosed. For example, the following steps are carried out for the treatment of chromium-based wastewater: firstly, hexavalent chromium is reduced to trivalent chromium. Natrium sulphite (Na2SO3), natrium bisulphite (NaHSO3) or SO2 gas are used as reduction products. The chromium is then precipitated in a weak alkali environment by forming chromium hydroxides, followed by the separation of the formed chromium hydroxides via, for example, sedimentation in a sedimentation tank.
Another application is the degradation of cyanide. Cyanide (CN-) present in wastewater is converted into cyanate by powerful oxidation products at pH 10. The used oxidation products could include lime chloride and natrium-hypochlorite (NaOCl). This substance is then separated using precipitation or filtration. Like cyanide, analogue reactions are used to detoxify wastewater containing nitrite, sulphide or sulphite.
Detoxification can also be combined with neutralisation and drainage of the formed precipitate in a DND system (Detoxification-Neutralisation-Drainage). Hydroxide sludge is drained in this system, often with a chamber filter compressor.
New techniques for the detoxification of wastewaters include:
- The use of biological processes
- Advanced oxidation processes like Fenton, or UV light or even direct sunlight catalysed in combination with TiO2.
- Advanced reduction processes like the use of zerovalent iron.
Specific advantages and disadvantages
Detoxification of wastewater sometimes enables the wastewater to be reused or can be useful as a pre-treatment for biological water purification. A disadvantage is that the yield of the method above is less than 100%. This means that, in practice, a particular quantity of harmful components will still be present in the effluent. In some cases, the reactions can also be reversible by, for example, the presence of oxidants or reductants.
Application
Detoxification is often implemented in wastewater derived from the galvanic and chemical industry. Further, these techniques are also implemented in purifying water that is derived from soil purification.
Examples of the detoxification of inorganic compounds include:
- NaOCl oxidation for the detoxification of cyanide, nitrite, sulphide and sulphite in wastewater from surface treatments.
- The use of H2O2 (+UV or Fe) for the degradation of AOX, nitrite, sulphite and cyanides.
- O3 (+UV or H2O2) for the oxidation of cyanide.
Used reductants include FeSO4, NaHSO3 and NaS2O4. NH2SO3H can be used to reduce nitrite into nitrogen gas.
Boundary conditions
The effectiveness of detoxification is greatly determined by the specific application. The chemical parameters are particularly important for an effective result. These include the pH, the pollution content in the wastewater, disruptive elements, etc. In water containing cyanide and chromium, detoxification is needed for neutralisation. Alkali and acidic rinse water can be immediately neutralised.
Effectiveness
The yield of a detoxification system can amount to between 50 and 99.9%, depending on the component needing to be removed. The yield will be significantly improved by a combination of precipitation and good separation after detoxification. This is because precipitated components can no longer enter the solution after removal. Removal yields of around 95 to 98% for cyanides and metals can be realised using biological detoxification techniques. Removal yields for the sunlight technique amount to around 85 % COD removal after 12 hours of sunlight exposure and TiO2.
Support aids
The support aids are often chemicals, gases (ozone) and UV light.
Environmental issues
Toxic sludge and/or harmless sludge are the by-products of detoxification.
Costs
The costs for detoxification consist of the various support aids used with the technique, as well as the purifying components that must be implemented to achieve the required result.
The cost price for chemicals can vary between 0.2 and > 25 €/kg.
The annual costs for the detoxification of 200 l effluent per day using direct sunlight and TiO2 amount to an investment of € 230 and operational costs of € 120.
Comments
However, in a rather large number of cases, practical situations don’t support the theory. Thus lab research and/or pilot research are always recommended.
Complexity
The complexity of this technique is determined by the substances that need to be removed. The process may range from the dosage of a few chemical substances to a completely automated and integrated process, with the use of ozone, peroxide and a precipitation system.
Level of automation
The degree of automation is determined by the to-be-removed components and the extent to which they are present in the wastewater. Far-reaching automation is possible if an online measurement system is implemented. In other cases, more intensive follow-up and modification will be necessary.
References
- AEA Technology, Manual of Effluent Process Technology, Environmental & Process Engineering Department, Harwell (GB), 1991
- Baeyens J., Hosten L. and Van Vaerenbergh E., Wastewater purification, Environment Foundation - Kluwer Editorial, 1995
- EIPPCB, Reference Document on BAT in Common Waste Water and Waste Gas Treatment / Management Systems in the Chemical Sector, draft February 2009 (revision upon release)
- Koot A.C.J., Treatment of wastewater, 2nd edition, Waltman Publishers, Delft, 1980
- VOM (Association for Surface Techniques on Materials), Wastewater Treatment Handbook, derived from surface treatments on metals, 1997
- Geomicrobiology Journal, Volume 8 , Issue 3 & 4 July 1990 , pages 241 – 249
- International Journal of Natural Sciences and Engineering 1:4 2008
- VITO-SCT, revision of technical notes WASS, 2009
Version February 2010

