Principle
- Insertion of zero-valent iron, or another granular or powder zero-valent metal/alloy with which the pollution is chemically reduced or precipitated
- Insertion of a chemical reactant, other than zero-valent iron, with which the pollution is chemically reduced or precipitated
- Application of zero-valent iron, or another granular zero-valent metal/alloy, possibly mixed with filter sand, as a reactive zone/wall, in which the pollution is chemically reduced or precipitated (plume management)
Techniques:
- injecting in vertical filters
- injecting in horizontal filters
- injecting in/applying in excavation
- injecting with direct push
- infiltration into excavated area
- infiltration in open canal
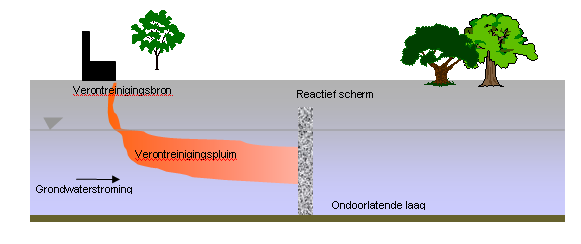
Figure: Diagram of In situ chemical reduction in a permeable reactive wall.
In-situ chemical reduction is a technique which is aimed at:
- (1 ) the in-situ destruction of pollutants by injecting a chemical reduction agent in the soil, normally the water-saturated zone. Possible reduction agents include dithionite, and zero-valent metal powders (in slurry) like iron and zinc.
- (2) same as (1), though in a permeable reactive barrier (PRB) configuration, aimed at managing groundwater plumes. In this case, zero-valent granular iron is normally used, possibly mixed with filter sand.
Field of application and application conditions
In-situ chemical reduction is suitable for treating
It is not/less suitable for
The technique is namely suitable for good to average permeable soil
A PRB can be implemented as a continuous wall, but also in “Funnel & Gate” configuration: thus a large part of the wall is permeable (the funnel) and reactive permeable zones are only placed at one (place) or a few places (the gates).
The workings and the required quantities (or wall thickness) must be determined on the basis of laboratory tests and calculations (possibly groundwater modelling).
Other important design factors/boundary conditions include:
- Total pollution content;
- In-situ chemical reduction can often be combined with in-situ reductive bio-remediation (injection of an organic substrate);
- Zero-valent iron, which comes into contact with water, oxidises whereby iron oxides are formed. Other precipitates are also formed (sulphide, carbonates…) along with hydrogen gas, which can over time lead to a sharp reduction in the wall’s permeability;
- A PRB is a management technique. In other words, the desired life-span is often long; currently there is uncertainty about the effectiveness of PRB’s in the long term;
- Zero-valent metals which come into contact with water lead to a sharp increase in pH;
- Chemical reduction agents such as dithionite are themselves potentially harmful to humans and the environment (overdosing must be avoided).
For further technical information about in-situ chemical reduction in PRB’s, please refer to the Code of Good Practice.
Costs
The cost of a preliminary investigation at laboratory scale, to determine the feasibility of this technique, amounts to around 5000 euros for simple batch tests, and up to 40,000 euros for more complicated column tests (VITO, 2008). Pilot tests (injection of chemical reduction agents) cost ca. 20-40 K€.
The installation costs for a PRB are greatly determined by the required length, depth and thickness, and by potential infrastructure conditions (nearby constructions, etc.). An estimate for a continuous wall amounts to 200 – 400 euros per m².
Environmental damage and to-be-implemented measures
If dimensioning is correct (full use of implemented oxidants in a polluted soil zone) then there is hardly any environment damage. Zero-valent iron is converted into iron oxides which are also naturally present in the soil. The increased pH drops to the normal level as one goes downstream, due to the natural pH buffering capacity of soils.
In PRB’s with zero-valent metals, it may be necessary to dispose of the produced hydrogen in a controlled manner. An explosion risk may be created if hydrogen is allowed to accumulate in the presence of oxygen.

